(a) Calculate the amount of energy released by the fusion of 1 g of deuterium according to...
Question:
(a) Calculate the amount of energy released by the fusion of 1 g of deuterium according to the nuclear reactions indicated in (4.1), considering as end products 4He, 1H, and 1n. Assume that the two possible results shown in (4.1), for the reaction 2H + 2H, occur with equal probabilities.
(b) How much energy can be released from the fusion of all the deuterium that exists in one liter of ordinary water? Compare this much energy with the energy obtained from the combustion of one liter of gasoline.
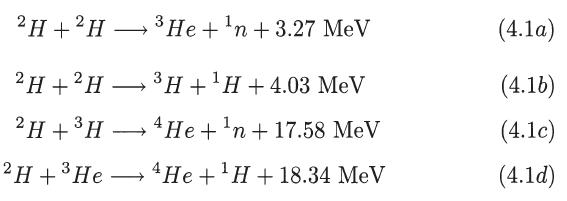
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





