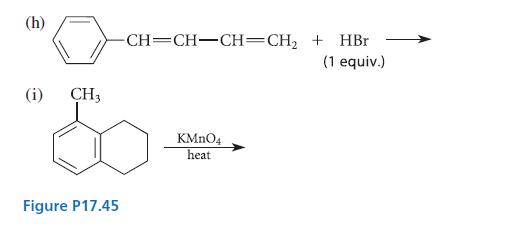
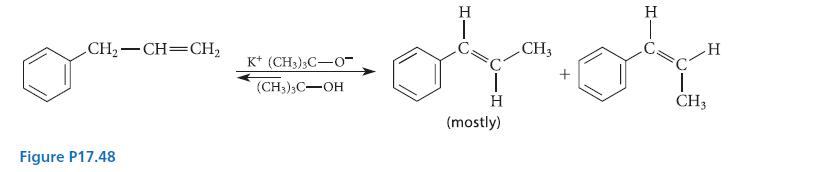
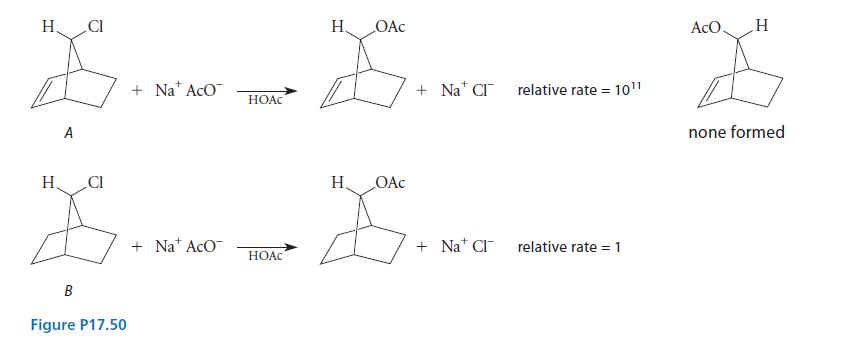
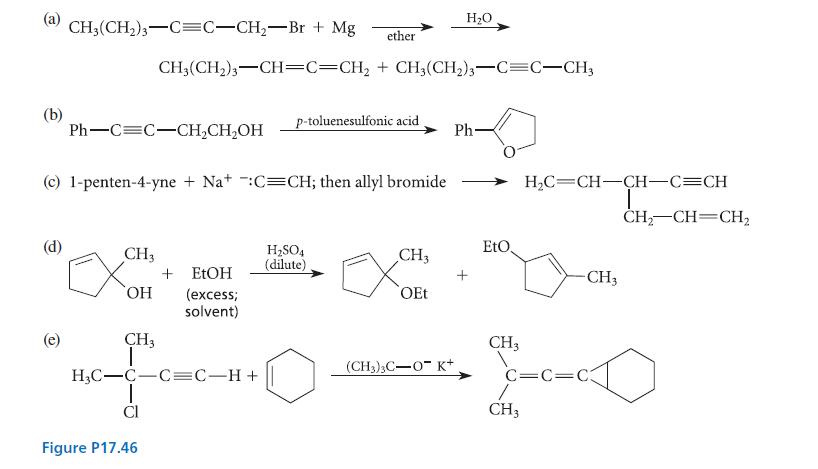
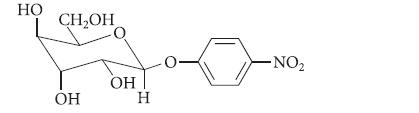
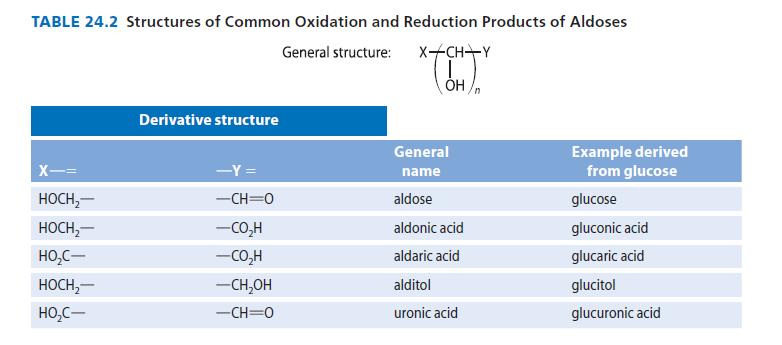
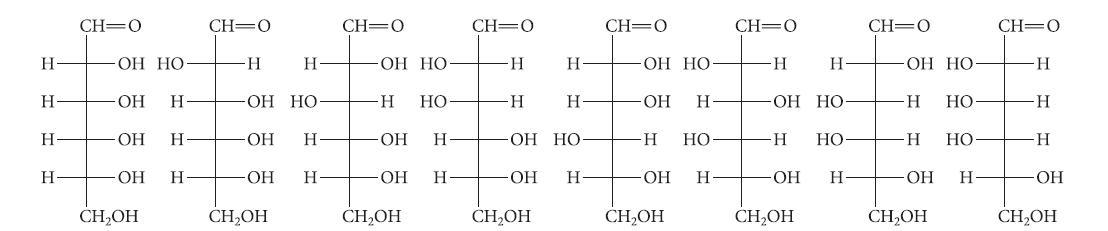
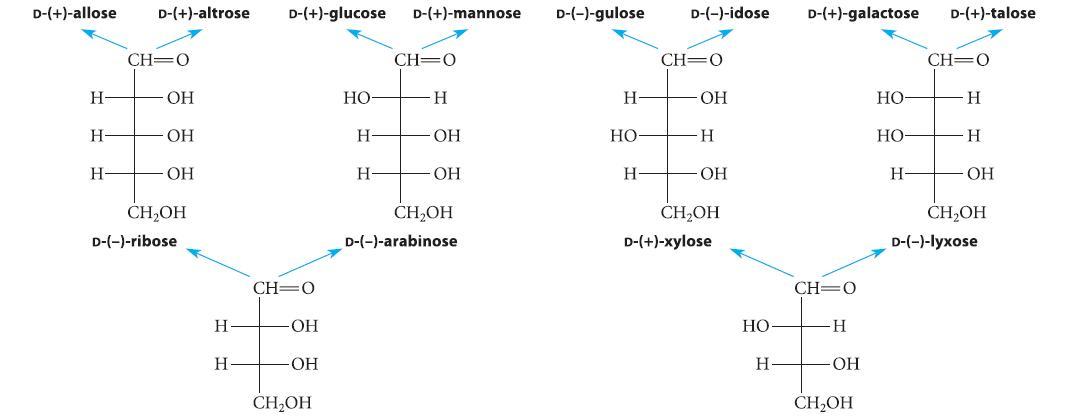
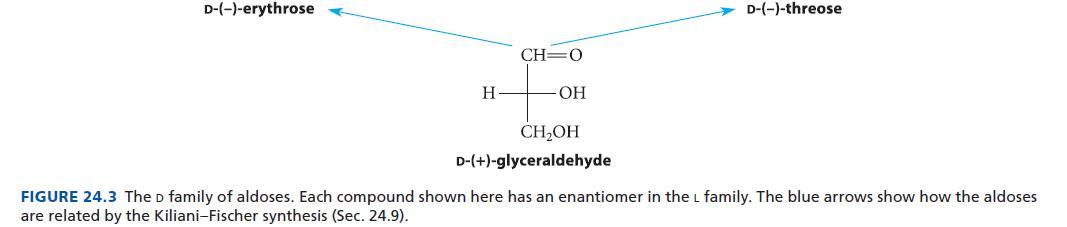
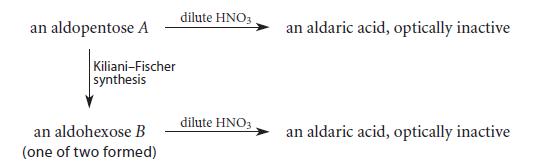
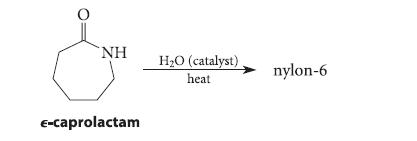
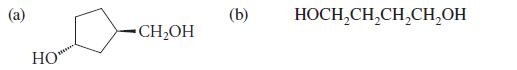Organic Chemistry 6th Edition Marc Loudon, Jim Parise - Solutions
Unlock the full potential of "Organic Chemistry 6th Edition" by Marc Loudon and Jim Parise with our comprehensive resources. Dive into a world of knowledge with our online answers key, solutions pdf, and detailed step-by-step answers. Whether you're tackling solved problems or exploring chapter solutions, our expertly crafted materials, including the instructor manual and test bank, offer invaluable insights. Perfect for students and educators alike, this textbook's questions and answers are now easily accessible for free download. Elevate your understanding of organic chemistry with these essential tools.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


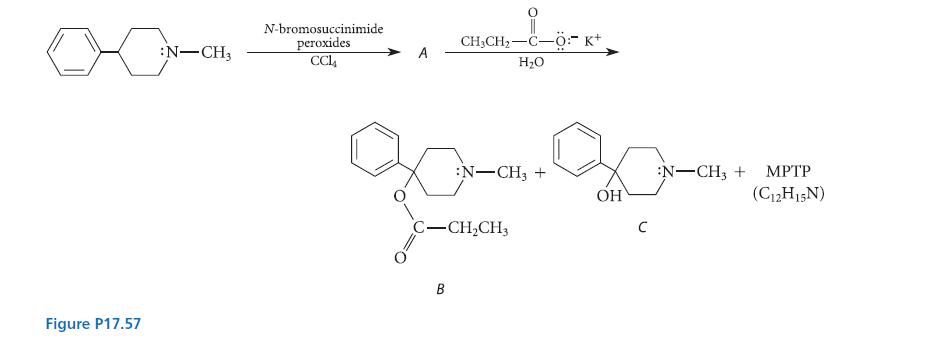
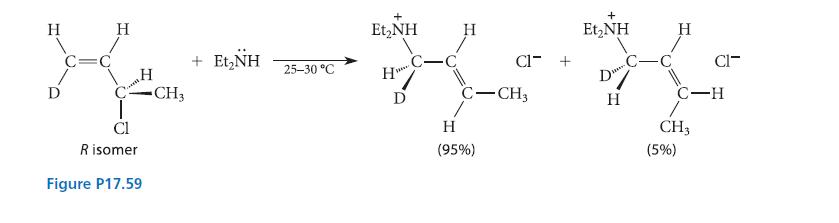
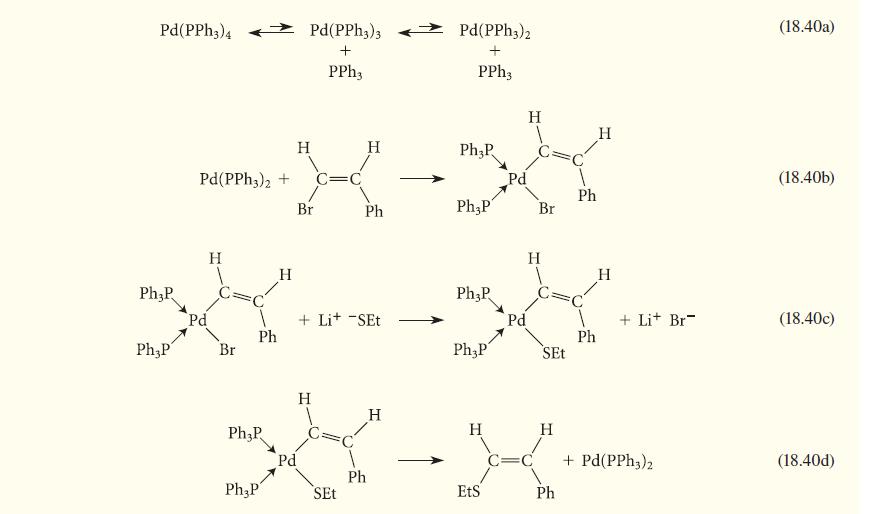
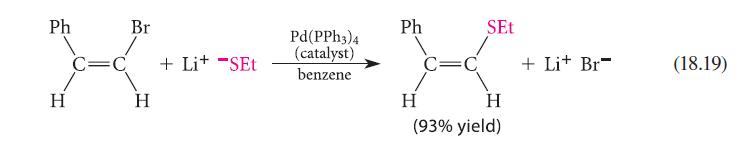
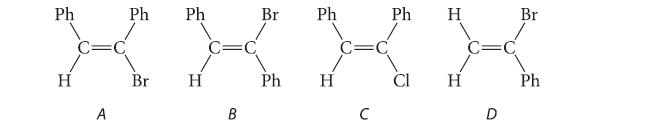
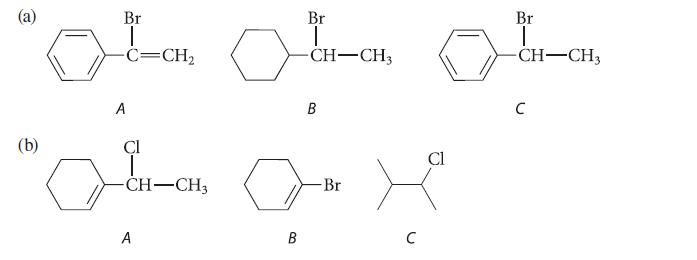
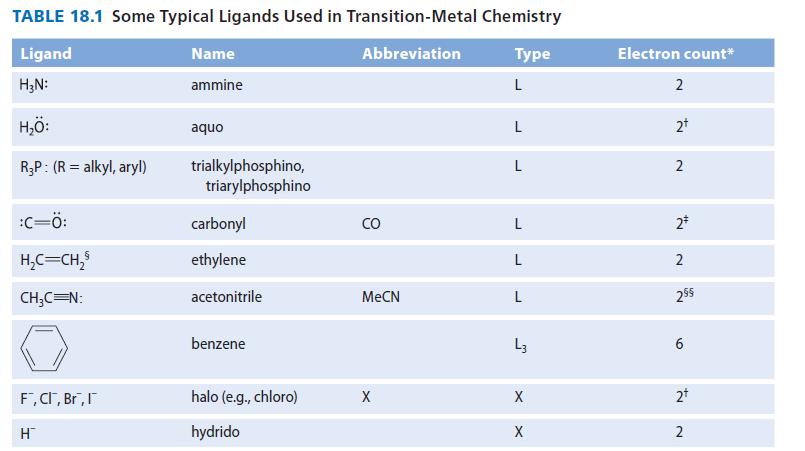
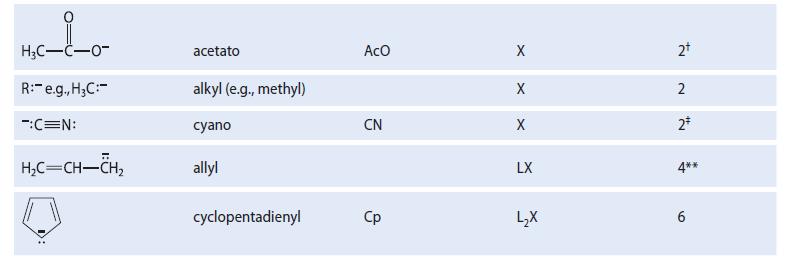
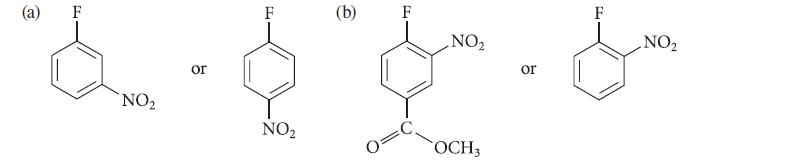

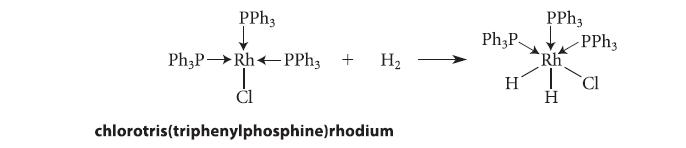
![[TcL6], where L = +:C=N- "sestamibi" OCH3 N-(2-methoxyisobutyl) isocyanide (MIBI)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/6/8/1/238656d98565c0571701681235537.jpg)
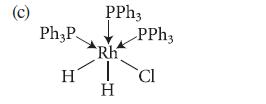
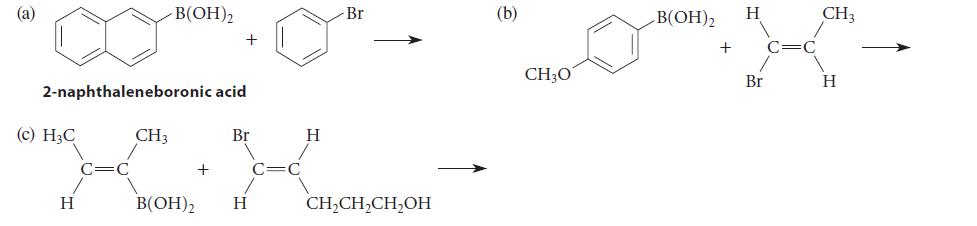
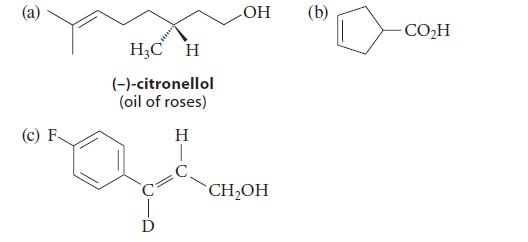
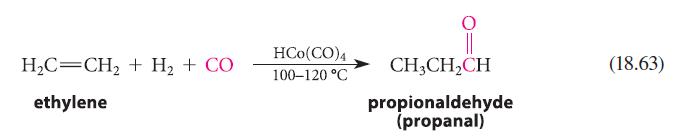
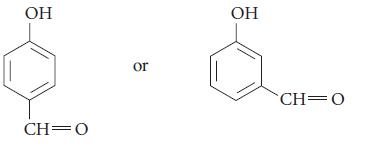
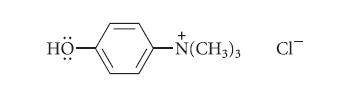
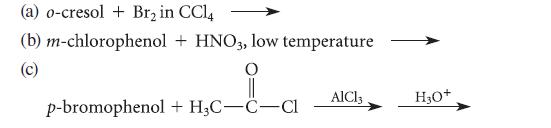
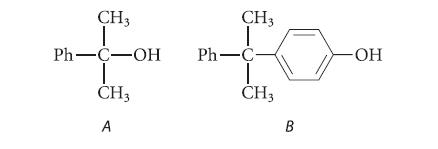
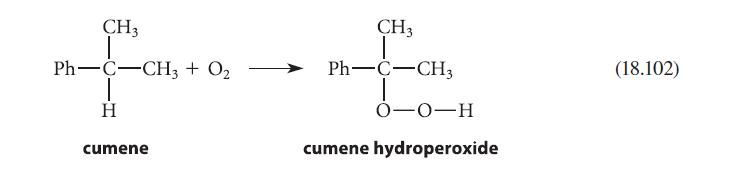
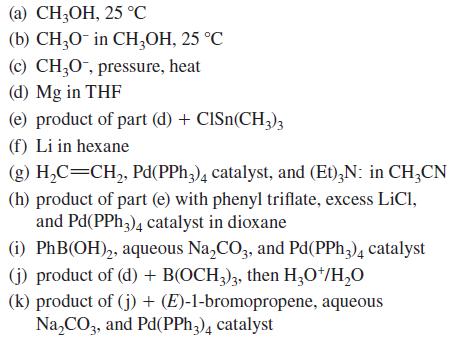
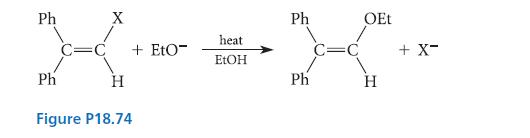
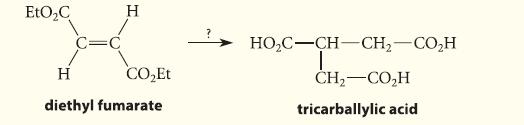
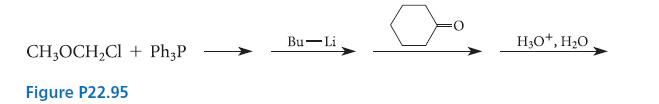
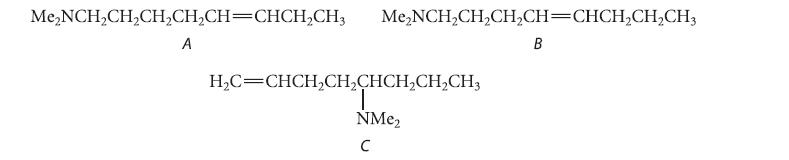
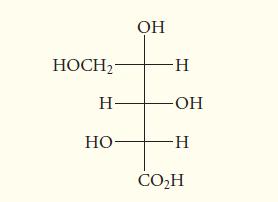
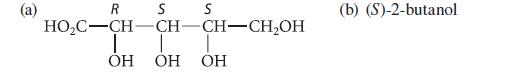
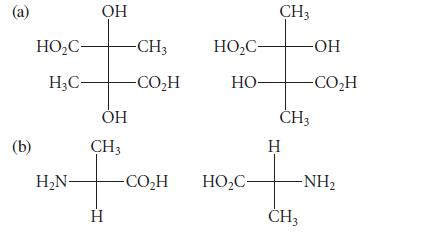
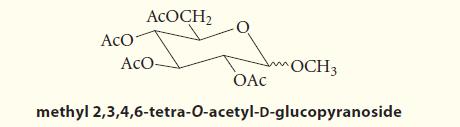
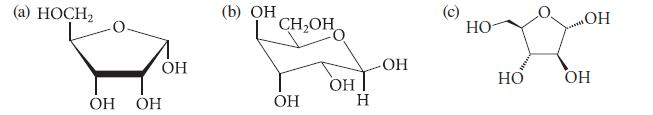
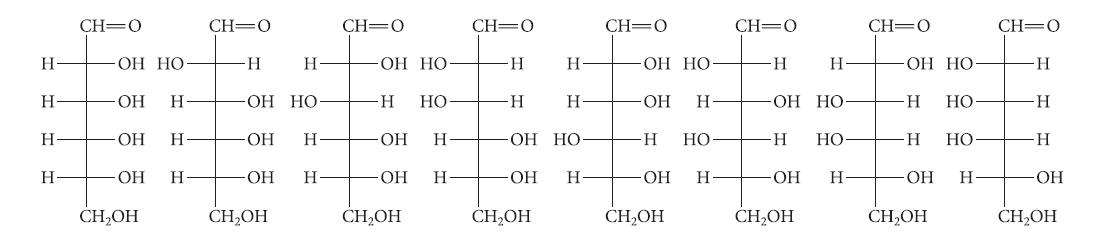
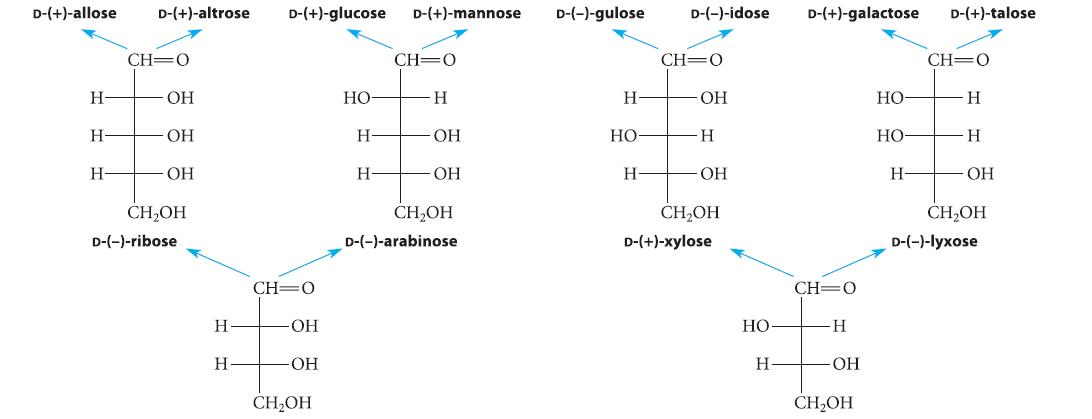
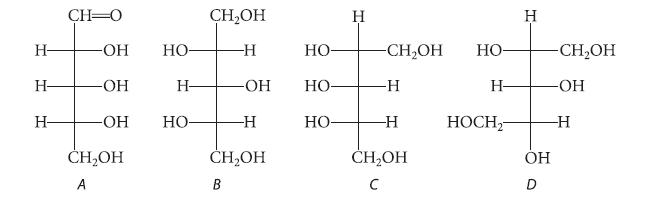
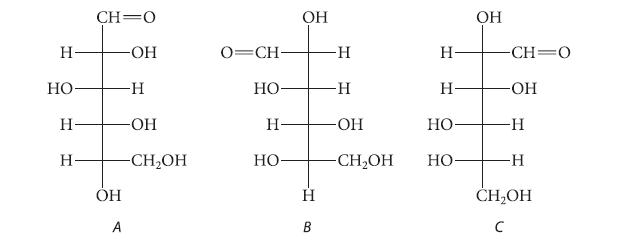
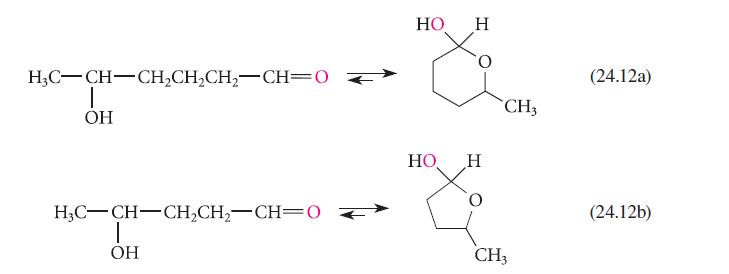
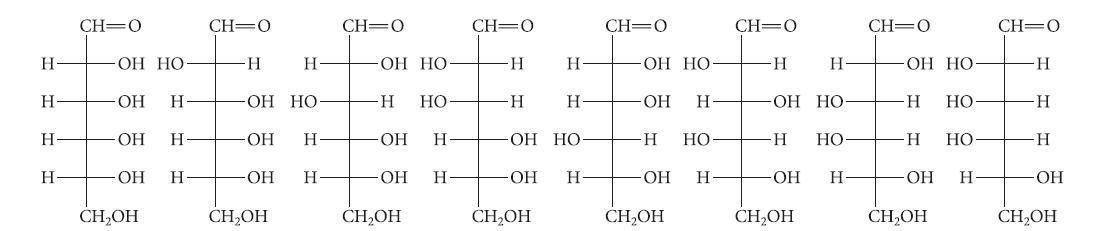
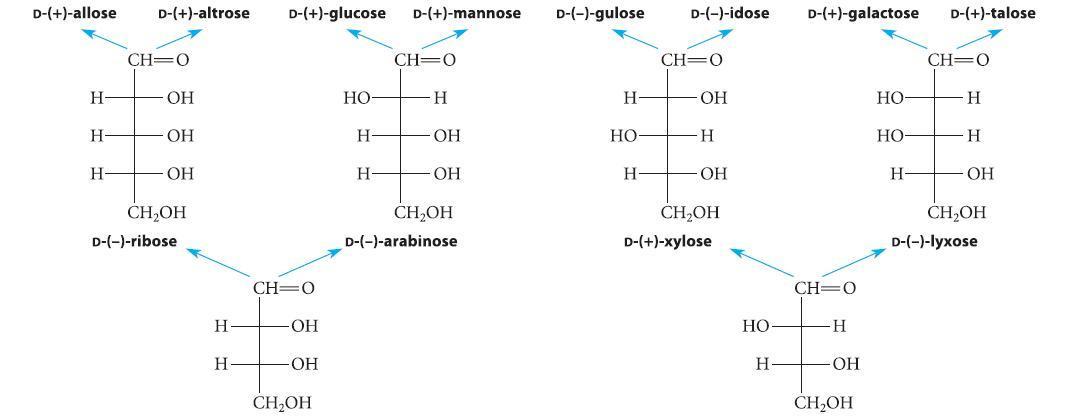
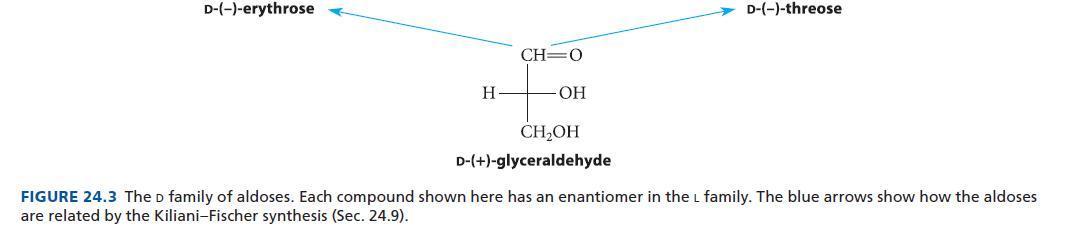
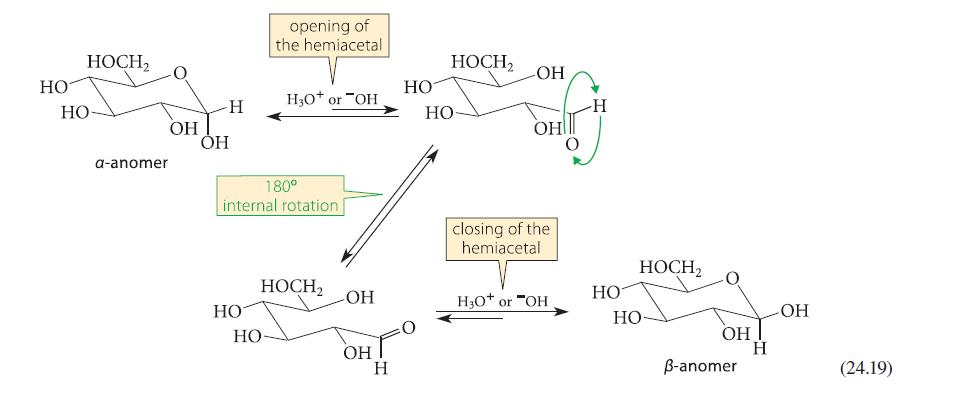
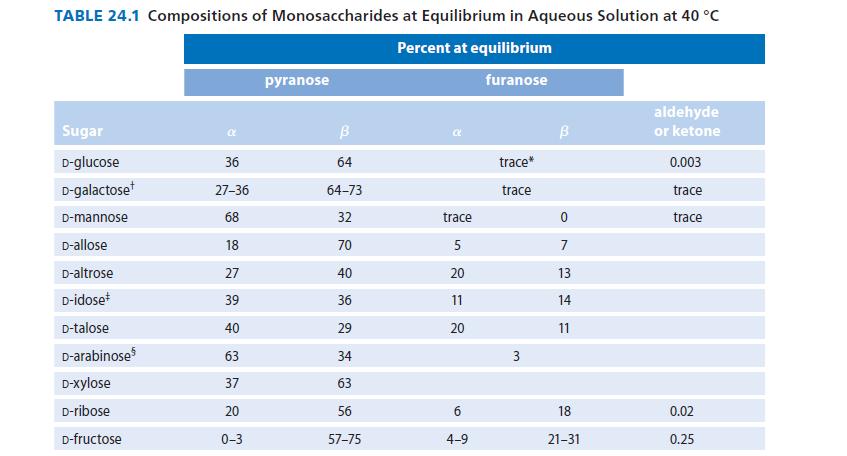
![HO HOCH HO OH -H OH a-anomer [a] =+112 degrees ml g-1 dm- acid or base H20 HOCH HO OH equilibrium mixture:](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/9/3/2/84265716f2a8015e1701932840806.jpg)