What is the oxidation state of the metal in the starting material in the following reaction? How
Question:
What is the oxidation state of the metal in the starting material in the following reaction? How does it change, if at all, as a result of the reaction? Is this reaction an oxidation, a reduction, or neither?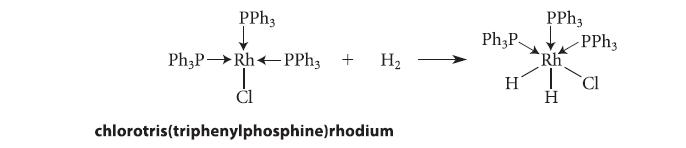
Transcribed Image Text:
PPh3 Ph3P→→Rh PPh3 + H₂ I CI chlorotris(triphenylphosphine) rhodium Ph3P H PPh3 Rh H -PPh3 CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
With one Xtype ligand CI and zero charge oxidation state of rhodiu...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1934+ Reviews
4278+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose that a life aged 30 arranged an insurance with the following parameters. If death occurs in the first 20 years, 10,000 is paid. Otherwise, 20,000 is paid. Moreover, it was arranged with the...
-
Consider a thin square sheet of side L and thickness t, made of a material of resistivity p. The resistance between two opposite faces, shown by the shaded areas in the figure is A) directly...
-
You have decided to purchase additional memory for your computer in order to better support the latest version of the Windows operating system. At the local computer store, you notice that not only...
-
Consider the generalized externality problem. Assume the damage and cost functions are given by: (a) Determine the non-regulated level of E if the polluter has the right to pollute. (b) Determine the...
-
The mean lifetime of a pion traveling at high speed is measured to be 7.5 10 -8 s. Its lifetime when measured at rest is 2.6 10 -8 s. How fast is the pion traveling?
-
Identify which of the following expenditures is considered as a capital expenditure that must be capitalized (depreciated): (a) Purchase land to build a warehouse at $300,000. (b) Purchased a copy...
-
What is socialization? AppendixLO1
-
a. Firm D has net income of $83,700, sales of $2,790,000, and average total assets of $1,395,000. Calculate the firms margin, turnover, and ROI. b. Firm E has net income of $150,000, sales of...
-
Dakota Company experienced the following events during 2018: 1. Acquired $25,000 cash from the issue of common 2. Paid $10,000 cash to purchase land. 3. Borrowed $10,000 cash. 4. Provided services...
-
(a) What is the electron count for the Rh complex shown in Problem 18.9c? (b) Sestamibi (trade name Cardiolite ) is a complex of 99 Tc(I) (a radioactive -ray emitter) that is widely used for cardiac...
-
Calculate the oxidation state of the metal in each of the following complexes. (a) O Mn-O- permanganate (b) Pd(PPH3)4 tetrakis(triphenylphosphine) palladium (c) CpFe ferrocene
-
Increases in oil prices have been blamed for several recessions in developed countries. To quantify the effect of oil prices on real economic activity, researchers have done regressions like those...
-
If a change were made to Technical Spec 2 in the product's design, this would likely change the customer's opinion of which value feature the most? Quick Start Quick Start QFD Matrix 1 = Strong...
-
You are a quality management consultant for the Beserk Tennis Ball Company. Beserk is redesigning its current model of tennis ball, and you are asked to use QFD analysis to make suggestions about...
-
You are reviewing a tender evaluation that is to be awarded on lowest total price. The bid evaluations follow: To which company should the contract be awarded? Company Capital Cost Maintenance...
-
You have invited four companies to bid on a consulting project. All four companies answered your invitation to tender, but the bids vary in the number of hours each company estimates will be required...
-
Boston Cycles inventory data for the year ended December 31, 2011, follow: Assume that the ending inventory was accidentally overstated by $2,200. Requirement 1. What are the correct amounts for cost...
-
These data are obtained for photoelectric stopping potentials using light of four different wavelengths. (a) Plot a graph of the stopping potential versus the reciprocal of the wavelength. (b) Read...
-
Write a paper detailing a geographic information system (GIS) of your own design that would utilize data in an original manner.
-
Tri-butyltin hydride (Bu3SnH) is used synthetically to reduce alkyl halides, replacing a halogen atom with hydrogen. Free-radical initiators promote this reaction, and free-radical inhibitors are...
-
When healthy, Earth's stratosphere contains a low concentration of ozone (O3) that absorbs potentially harmful ultraviolet (UV) radiation by the cycle shown at right. Chlorofluorocarbon refrigerants,...
-
Deuterium (D) is the hydrogen isotope of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C-D...
-
September 23 for $1,050 each. On December 24 , it sold one of the diamonds that was purchased on July 9 . Using the specific identification method, its ending inventory (after the December 24 sale)...
-
Madsen Motors's bonds have 13 years remaining to maturity. Interest is paid annually, they have a $1,000 par value, the coupon interest rate is 8%, and the yield to maturity is 10%. What is the...
-
Builder Products, Incorporated, uses the weighted - average method in its process costing system. It manufactures a caulking compound that goes through three processing stages prior to completion....

Study smarter with the SolutionInn App


