(a) Triphenylmethane [structure in part (b)] has a pK a of 31.5 and, although an alkane, it...
Question:
(a) Triphenylmethane [structure in part (b)] has a pKa of 31.5 and, although an alkane, it is almost as acidic as a 1-alkyne. (Most alkanes have pKa ≽ 55.) By considering the structure of its conjugate base, suggest a reason why triphenylmethane is such a strong hydrocarbon acid.
(b) Fluoradene is structurally very similar to triphenylmethane, except that the three aromatic rings are “tied together” with single bonds. Fluoradene has a pKa of 11. Suggest a reason why fluoradene is much more acidic than triphenylmethane.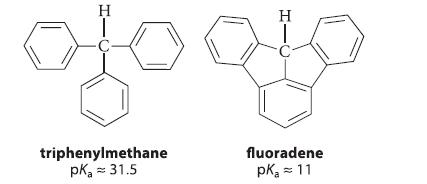
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





