The amount of anti-addition in the chlorination of alkenes varies with the structure of the alkene, as
Question:
The amount of anti-addition in the chlorination of alkenes varies with the structure of the alkene, as shown in the following table.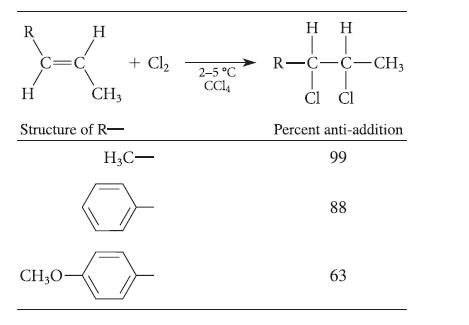
Suggest a reason for the variation in the stereochemistry of addition as the alkene structure is varied.
Transcribed Image Text:
R C=C H CH3 Structure of R- H CH₂0- + Cl₂ H3C- 2-5 °C CC14 HH IT R-C-C-CH3 CI CI Percent anti-addition 99 88 63
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
Section 78C shows that anti stereochemistry is one of the major pieces of evidence that a bromonium ...View the full answer

Answered By

YOGENDRA NAILWAL
As I'm a Ph.D. student, so I'm more focussed on my chemistry laboratory. I have qualified two national level exams viz, GATE, and NET JRF (Rank 68). So I'm highly qualified in chemistry subject. Also, I have two years of teaching experience in this subject, which includes college teacher as well as a personal tutor. I can assure you if you hire me on this particular subject, you are never going to regret it.
Best Regards.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The kinetic data for the radical chain chlorination of several cycloalkanes (see the adjoining table) illustrate that the C-H bonds of cyclopropane and, to a lesser extent, cyclobutane are somewhat...
-
In fatty-acid synthesis, malonyl-CoA, rather than acetyl-CoA, is used as a condensing group. Suggest a reason for this.
-
The critical temperature (K) and pressure (atm) of a series of halogenated are as follows: (a) List the intermolecular forces that occur for each compound. (b) Predict the order of increasing...
-
Why does allocating an array of length \(n\) take time proportional to \(n\) ?
-
The frequency of revolution of an electron in a circular orbit of radius r is frev = v/2r, where v is the speed. (a) Show that in the nth stationary state (b) Show that when n 1 = n, n 2 = n 1, and...
-
Using the Hospital database, construct a 90% confidence interval to estimate the average census for hospitals. Change the level of confidence to 99%. What happened to the interval? Did the point...
-
What is the difference between a work group and a real team? AppendixLO1
-
On January 1, 2017, Spring Fashions Inc. enters into a contract with a southeast retail company to provide 500 dresses for $ 62,500 ($ 125 per dress) over the next 10 months. On October 1, 2017,...
-
Lets consider the key topics learned in class, including: Financial Statements, Cash Flow, and Taxes Financial Assets and Inventories Global Business and Accounting Capital Budgeting and Capital...
-
(a) For each of the two reactions shown in Fig. P17.58, suggest a mechanism that is consistent with all of the experimental facts given. Experimental observations: (1) Both reactions conform to the...
-
(a) Triphenylmethane [structure in part (b)] has a pK a of 31.5 and, although an alkane, it is almost as acidic as a 1-alkyne. (Most alkanes have pK a 55.) By considering the structure of its...
-
In 2007, Peggy, a widow, places $3 million in trust, life estate to her children, remainder to her grandchildren, but retains the right to revoke the trust. In 2011, when the trust is worth $3.1...
-
1 . Journalize the following transactions: ( a ) Issued 1 , 0 0 0 shares of $ 1 0 par common stock at $ 5 9 for cash. ( b ) Issued 1 , 4 0 0 shares of $ 1 0 par common stock in exchange for equipment...
-
Using alpha .05, determine if moving to a larger enclosure decreased tiger anxiety levels. You should first calculate the difference (After - Before) Tiger Before Anthony 45 45 Banthony 56 After 38...
-
Cyclohexane (C 6 H 12 ) is produced by mixing Benzene and hydrogen. A process including a reactor, separator, and recycle stream is used to produce Cyclohexane. The fresh feed contains 260L/min C 6 H...
-
Suppose the city is undergoing severe ination. Specifically, both goods prices have risen by 10%. What percentage of a raise in the wage rate should Alex request from her boss, for her to maintain...
-
1. An iron cube of mass 0.55 kg is raised to a temperature of 100C by being placed in boiling water for 5 minutes. It is then removed and transferred immediately to an aluminium calorimeter filled...
-
By directly substituting the values of the fundamental constants, show that the ground state energy for hydrogen in the Bohr model E1 = me k2 e4 /(22) has the numerical value 13.6 eV.
-
Describe the Operations (+,,*,/) that can cause negligible addition (NA), error magnification (EM), or subtractive cancellation (SC) in calculating ?((x^2)+1) - x . Give the range of where they might...
-
To show that (R)-2-butyl (R, R)-tartrate and (S)-2-butyl (R,R)-tartrate are not enantiomers, draw and name the mirror images of these compounds.
-
The following four structures are naturally occurring optically active compounds. Star the asymmetric carbon atoms in these structures. CHO H CH, COOH OH OH H,N H serine erythrose menthol camphor
-
For each structure, 1. Star (*) any asymmetric carbon atoms. 2. Label each asymmetric carbon as (R) or (S). 3. Draw any internal mirror planes of symmetry. 4. Label the structure as chiral or...
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


