Complete the following Pd(0)-catalyzed Suzuki reactions by giving the coupling product. For parts (b) and (c), include
Question:
Complete the following Pd(0)-catalyzed Suzuki reactions by giving the coupling product. For parts (b) and (c), include the stereochemistry of the products.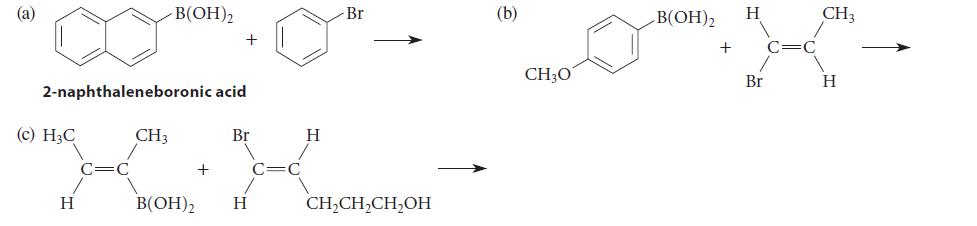
Transcribed Image Text:
(a) 2-naphthaleneboronic acid (c) H₂C H -B(OH)2 CH3 B(OH)2 + + Br H C C H Br CH₂CH₂CH₂OH (b) CH3O B(OH)2 + H Br C CH3 H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
As illustrated in Eq 1849 text p 904 Suzuki coupling occurs w...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Complete the following Pd(0)-catalyzed Suzuki reactions by giving the coupling product. Include the stereochemistry of the products. BOH CH3 CH3O
-
Complete the reactions given in Fig. P22.81 by giving the major organic products. Explain your reasoning. NaOEt excess) EtOH H,o heat CHsI (c) CI NO, H ot, heat C CH LiAIH 2) HaO (e) CH Et CHCHCOEOH...
-
Explain how has the business world made a huge shift with regard to the physical workplace and communication? How has that affected the role of managers?
-
Consider a regulators objective formulized as: (a) Show that at most one of the four constraints (incentive compatibility and participation) can be binding in this case, that the high cost firms fee...
-
Show that when V < < c the transformation equations for x, t, and u reduce to the Galilean equations.
-
Rework Problem 12.20, assuming the following additional information: The old switching system has been fully depreciated. The new system falls into the five-year MACRS property class. The companys...
-
Describe the emotional intelligence competency called social skills. AppendixLO1
-
AAA Appliances Inc. has two production departments. The nature of the process is such that no units remain in process in Finishing at the end of the period. At the beginning of the period, 10,000...
-
Obtain the forensic accountant's report located by performing a Google search using "forensic accountant slams high costs, low competition" as a search term. A link to the white paper is available at...
-
The product of a Heck reaction is, like the starting material, an alkene. Why doesnt a Heck reaction of the product compete with the reaction of the starting alkene?
-
When iodobenzene and propene are subjected to the conditions of the Heck reaction, two constitutionally isomeric products are formed. What are they? Why are two products formed?
-
Name the six relational operators and state the purpose of each operator.
-
Lennys Limousine Service (LLS) is considering the purchase of two Hummer limousines. Various information about the proposed investment follows: Required: Help LLS evaluate this project by calculating...
-
Lancer Corp. has the following information available about a potential capital investment Required: 1. Calculate the projects net present value. 2. Without making any calculations, determine whether...
-
Woodchuck Corp. is considering the possibility of outsourcing the production of upholstered chair pads included with some of its wooden chairs. The company has received a bid from Padalong Co. to...
-
Woodchuck Corp. is considering eliminating a product from its line of outdoor tables. Two products, the Oak-A and Fiesta tables, have impressive sales. However, sales for the Studio model have been...
-
Suppose that Flyaway Company also produces the Windy model fan, which currently has a net loss of \($40,000\) as follows: Eliminating the Windy product line would eliminate \($20,000\) of direct...
-
A 100-W light bulb radiates visible light at a rate of about 10 W; the rest of the EM radiation is mostly infrared. Assume that the light bulb radiates uniformly in all directions. Under ideal...
-
Refrigerant-134a enters an adiabatic compressor as saturated vapor at 120 kPa at a rate of 0.3 m3/min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 80 percent,...
-
Consider the following reaction-energy diagram. (a) Label the reactants and the products. Label the activation energy for the first step and the second step. (b) Is the overall reaction endothermic...
-
Draw a reaction-energy diagram for a one-step exothermic reaction. Label the parts that represent the reactants, products, transition state, activation energy, and heat of reaction.
-
Draw a reaction-energy diagram for a two-step endothermic reaction with a rate-limiting second step.
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


