Give the product formed when each of the following alcohols is oxidized by dilute HNO 3 .
Question:
Give the product formed when each of the following alcohols is oxidized by dilute HNO3.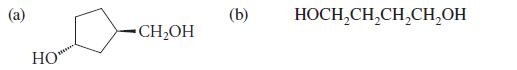
Transcribed Image Text:
HO CH₂OH (b) HOCH₂CH₂CH₂CH₂OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
As is the case with carbohydrates ...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the product formed when the following alcohols is oxidized by dilute HNO3. HOCH2CH 2CH2OH
-
Give the product formed when each of the following compounds undergoes an electrocyclic reaction a. Under thermal conditions b. Under photochemical conditions 1. 2. CH3 CH ,.
-
Give the major product formed when each of the following alcohols is heated in the presence of H2SO4; a. b. c. d. e. f. CH3CH2CH2CH2CH2OH CH CH3CH2C CHCH OH CH3 CHCH2CH3 OH CH,OH CH3 CH,CH2CH CCH OH...
-
We can measure how good a center Kevin Bacon is by computing each performer's Hollywood number or average path length. The Hollywood number of Kevin Bacon is the average Bacon number of all the...
-
A particle of rest mass M 0 decays into two identical particles of rest mass m 0 , where m 0 = 0.3M 0 . Prior to the decay, the particle of rest mass M 0 has an energy of 4M 0 c 2 in the laboratory....
-
How can you use information about a persons values to help you relate more effectively to him or her?
-
What is the probability that none of the 80 people would rate their financial shape as fair? (Make the assumption that the 500 people are represented by the pie chart.)
-
Munger.Com began operations on January 1, 2006. The company reports the following information about its investments at December 31, 2006: Required: a. Show how each of these investments are reported...
-
On January 1, 2014, BCD paid $600,000 for 60,000 shares of MNO's common which represents a 25 percent investment in MNO. BCD can exercise significant influence over MNO. BCD received a cash dividend...
-
Give Fischer projections for the aldaric acids derived from both D-glucose and L- gulose. What is the relationship between these structures?
-
An aldohexose A is either d-idose or d-gulose (see Fig. 24.3). It is found that a different aldohexose, L -(2)-glucose, gives the same aldaric acid as A. What is the identity of A? - - H CH=0 -OH HO...
-
Two infinitely long solenoids (seen in cross section) pass through a circuit as shown in Figure P31.49. The magnitude of B inside each is the same and is increasing at the rate of 100 T/s. What is...
-
Verify the results of Eq. (14.48) for the properties of the chiral projection operators. Data from Eq. 14.48 P = P+ P+ + P = 1 P_P+ P+P = 0 Py" = y P
-
Prove that the estimating equations in (11.13) are unbiased under MCAR, but are generally biased without the stringent MCAR assumption. (x) [y - f (xt;)] = 0, i=1 (11.13)
-
Refer to Figure 11.5: Which is the most expensive subcontract for this project? How much were the costs for the general contractor's crews for item 4? Figure 11.5 Division 1 2 3 4 5 6 7 Work Gen'l...
-
a. Using observations on the change in consumption \(D C_{t}=C_{t}-C_{t-1}\) and the change in income \(D Y_{t}=\) \(Y_{t}-Y_{t-1}\) from 1959Q3 to 2015Q4, obtained from the data file cons_inc,...
-
Water at \(20^{\circ} \mathrm{C}\) flows by gravity from a large reservoir at a high elevation to a smaller one through a 35-m-long, 5-cm-diameter cast iron piping system that includes four standard...
-
The particle in a box model is often used to make rough estimates of ground-state energies. Suppose that you have a neutron confined to a one-dimensional box of length equal to a nuclear diameter...
-
Subtract the polynomials. (-x+x-5) - (x-x + 5)
-
Suppose a mixture of AQC-amino acids is subjected to HPLC on a stationary phase that consists of C8-silica rather than Cl 8-silica; that is, the glass stationary phase (Eq. 26.34, p. 1293) contains...
-
Sometimes it is necessary in solid-phase peptide synthesis to use a resin linker that is more sensitive (that is, more reactive) to acid than the linker shown in Eq. 26.21 on p. 1285. The following...
-
Draw the structure of the major neutral form of each of the following peptides, G-D-G-L-F
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...

Study smarter with the SolutionInn App


