Draw at least two Fischer projections for each of the following molecules. S S R HOCCHCH CHCH,OH
Question:
Draw at least two Fischer projections for each of the following molecules.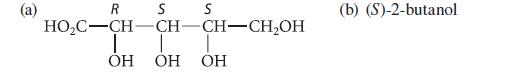
Transcribed Image Text:
S S R HOC–CH–CH CH–CH,OH I T T OH OH OH (b) (S)-2-butanol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
b Many Fischer projections are possible for a compound ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw, at least two Fischer projections for the following molecules. (s) - 2butanol
-
Write Fischer projections for each of the compounds of Problem 7.7. In Problem 7.7 Assign absolute configurations as R or S to each of the following compounds: a. b. c. H3C -CH2F CH3CH2...
-
For each of the following organic molecules draw a Lewis structure in which the carbon atoms are bonded to each other by single bonds: (a) C2H6, (b) C4H10, (c) C5H12. For (b) and (c), show only...
-
Integer sort. Write a linear-time filter that reads from standard input a sequence of integers that are between 0 and 99 and prints to standard output the same integers in sorted order. For example,...
-
Use Equations 39-21 and 39-25 to derive the equation E 2 = p 2 c 2 + (m 0 c 2 ) 2 . 39-21 Vi-ulc moc2 Vi-ulc E = K + m,c 39-25
-
Convince yourself that the natural filtration, F 2 generated by observing the events A 1 = {(H, H), (H, T)} and A 2 = {(H, T)}, has only eight elements.
-
12-10. A managers key task is to balance which four customer service factors against which six logistics cost factors?
-
Tyler Countys general fund starts the fiscal year 2020 with a $250,000 balance for net property taxes receivable. The balance is net of a $200,000 allowance for uncollectible taxes. The county also...
-
7 Following is information on two alternative investments being considered by Jolee Company. The company requires a 105 return from is investments PV 51. EVO 51 PVA of $1. and EVA 5.1 (Use...
-
Indicate whether the structures in each of the following pairs are enantiomers, diastereomers, or identical molecules. (a) (b) HOC- HC- HN- OH H OH CH3 -CH3 -CO,H -CO,H HOC- - HOC- CH3 CH3 H -OH...
-
When 1,5-dibromopentane reacts with ammonia, among several products isolated is a water- soluble compound A that rapidly gives a precipitate of AgBr with acidic AgNO 3 solution. Compound A is...
-
Future Value At age 25 you invest $1,500 that earns 8 percent each year. At age 40 you invest $1,500 that earns 11 percent per year. In which case would you have more money at age 65? (LG2)
-
Convert the following information into: a) a semantic net b) a frame-based representation A Ford is a type of car. Bob owns two cars. Bob parks his car at home.His house is in California, which is a...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Marriott: Marketing Research Leads to Expanded Offerings highlights Marriotts success in using...
-
The water level in a tank is \(20 \mathrm{~m}\) above the ground. A hose is connected to the bottom of the tank, and the nozzle at the end of the hose is pointed straight up. The tank cover is...
-
A simple experiment has long been used to demonstrate how negative pressure prevents water from being spilled out of an inverted glass. A glass that is fully filled by water and covered with a thin...
-
A golf ball is hit on a level fairway. When it lands, its velocity vector has rotated through an angle of 90. What was the launch angle of the golf ball? Pyo By Dyz =0 Uso Range R x max dya
-
It is sometimes said that, at absolute zero, all molecular motion, vibration, and rotation would cease. Do you agree? Explain.
-
The pendulum consists of two rods: AB is pin supported at A and swings only in the y-z plane, whereas a bearing at B allows the attached rod BD to spin about rod AB. At a given instant, the rods have...
-
In each reaction, label the reactants as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions. Draw in any nonbonding...
-
In 1934, Edward A. Doisy of Washington University extracted 3000 lb of hog ovaries to isolate a few milligrams of pure estradiol, a potent female hormone. Doisy burned 5.00 mg of this precious sample...
-
The pKa of ascorbic acid (vitamin C, page 2) is 4.17, showing that it is slightly more acidic than acetic acid (CH3COOH, pKa, 4.74). (a) Show the four different conjugate bases that would be formed...
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


