Suggest an alkene metathesis reaction that would yield each of the following compounds as a major product.
Question:
Suggest an alkene metathesis reaction that would yield each of the following compounds as a major product.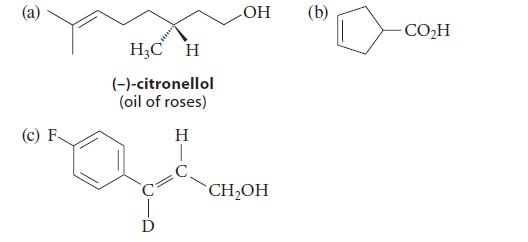
Transcribed Image Text:
(c) F H₂C H (-)-citronellol (oil of roses) H D OH CH₂OH (b) CO₂H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a b HC H citronellol oil of roses OH ...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In each case, give the structure of an eight-carbon alkene that would yield each of the following compounds (and no others) after treatment with ozone followed by dimethyl sulfide. (b)...
-
Each of the following compounds is an aromatic compound bearing a substituent that we did not discuss in this chapter. Using the principles that we discussed in this chapter, predict the major...
-
Write structural formulas for all the alkene products that could reasonably be formed from each of the following compounds under the indicated reaction conditions. Where more than one alkene is...
-
Consider two polluting firms with cost functions C j (x j ,e j ). The inverse demand function is P(X). Firms engage in negotiations about the permit allocation and a transfer price in a first stage...
-
What is the separation distance between clocks A and B according to the observer inS? y:V= 0.6c -100 cmin- A' Flashbulb
-
You borrowed $4,000 to finance your educational expenses at the beginning of your junior year of college at an interest rate of 9% compounded annually. You are required to pay off the loan with five...
-
Describe the democratic leadership style. AppendixLO1
-
Sharp Motor Company has two operating divisionsan Auto Division and a Truck Division. The company has a cafeteria that serves the employees of both divisions. The costs of operating the cafeteria are...
-
I need help on this question. I keep trying $1273.62 as the answer and it wont work. Can anybody figure out the problem? Excel Online Structured Activity: Bond valuation You are considering a...
-
Suggest a mechanism for the oxo reaction (Eq. 18.63) involving intermediates that are consistent with the 16- and 18-electron rules. H,C=CH2 + H2 + CO ethylene HCO (CO)4 100-120 C CHCHCH...
-
Give the structure of the major product formed in each case when the reactant(s) shown undergo alkene metathesis in the presence of an appropriate ruthenium catalyst. (a) CHOH T HC=CHCHCHCHCHCH=CH x...
-
When the owner of a gas station sets the price of 1 gallon of unleaded gasoline at $2.10, she can sell approximately 1500 gallons per day. When she sets the price at $2.25 per gallon, she can sell...
-
Refer to the information presented in M7-9. Suppose that Juanita has developed a rectangular, medium-size ceramic pot. It requires 3 hours of kiln time; however, two medium-size pots can fit m the...
-
Eclipse Company manufactures a variety of sunglasses. Production information for its most popular line, the Total Eclipse (TE), follows: Suppose that Eclipse has been approached about making a...
-
Sunblocker Corp. makes several varieties of beach umbrellas and accessories. It has been approached about producing a special order for custom umbrellas. The special-order umbrellas with the Randolph...
-
Sunblocker Corp. is considering eliminating a product from its Happy Sand line of beach umbrellas. This collection is aimed at people who spend time on the beach or have an outdoor patio near the...
-
Suppose that annual demand for a certain item has decreased dramatically this year, although the store that stocks this item has not updated its inventory policy, so the store is still using the same...
-
Follow the steps outlined in this problem to estimate the time lag (predicted classically but not observed experimentally) in the photoelectric effect let the intensity of the incident radiation be...
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Why do you think Google was adamant about not wanting to supply information requested by the government concerning the Child...
-
Draw the two chair conformations of each compound and label the substituents as axial and equatorial. In each case, determine which conformation is more stable. (a) Cis-1-ethyl-2-isopropylcyclohexane...
-
Using what you know about the conformational energetics of substituted cyclohexanes, predict which of the two decalin isomers is more stable. Explain your reasoning.
-
Convert each Newman projection to the equivalent line-angle formula, and assign the IUPAC name. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) CH2CH3 CH2CH3 CH3 CH CH3 Br CH3 CH2CH3 CH2CHs Cl CH CH(CH32...
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


