Suggest a mechanism for the oxo reaction (Eq. 18.63) involving intermediates that are consistent with the 16-
Question:
Suggest a mechanism for the oxo reaction (Eq. 18.63) involving intermediates that are consistent with the 16- and 18-electron rules.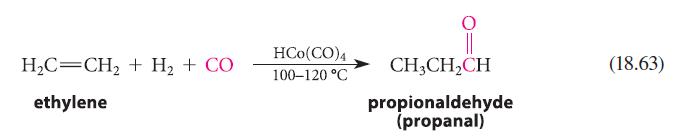
Transcribed Image Text:
H,C=CH2 + H2 + CO ethylene HCO (CO)4 100-120 °C CH₂CH₂CH propionaldehyde (propanal) (18.63)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The classifications of the various steps are 1 Substitution of one Ltype ligand for ano...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest a mechanism for the free-radical addition of HBr to cyclohexene initiated by AIBN. Show the initiation and propagation steps.
-
When acetoacetic acid is decarboxylated in the presence of bromine, bromoacetone is isolated (see Fig. P22.68). The rate of appearance of bromoacetone is described by the following rate law: (The...
-
Suggest a mechanism for each of the following reactions that accounts for both products. H,so, CH,CH CHCH,OH HBr CH,CH CHCH BrCH,CHCH CH2 (84%) Br (16%)
-
Consider the energy sector consisting of J firms where energy producers are characterized by their cost functions C j (x j ,e j ). The firms are subject to an emission trading system with a total...
-
As the light pulse from the flashbulb travels toward A with speed c, A travels toward C with speed 0.6c. Show that the clock in S reads 25 min when the flash reaches A. y:V= 0.6c -100 cmin- A'...
-
A loan of $20,000 is to be financed to assist a persons college education. Based upon monthly compounding for 48 months, the endofthemonth equal payment is quoted as $520. What nominal interest rate...
-
Describe the affiliative leadership style. AppendixLO1
-
Sound Investments, Inc. is a large retailer of stores equipment. The controller is about to prepare the budget for the first quarter of 20x2. Past experience has indicated that 75 percent of the...
-
Problem: Module 7 Textbook Problem 2 Learning Objective: 7-4 Explain the flow-through of partnership items to the partners This year, Herb Partnership generated $780,000 ordinary business Income....
-
Which of the two phenols in each set is more acidic? Explain. (a) 2,5-dinitrophenol or 2,4-dinitrophenol (b) phenol or m-chlorophenol (c) OH CH 0 or OH CH=0
-
Suggest an alkene metathesis reaction that would yield each of the following compounds as a major product. (c) F HC H (-)-citronellol (oil of roses) H D OH CHOH (b) COH
-
Using the Competing Values Framework Model, which the four different kinds of organization culture best fits the culture of Createasphere? Support your answer.
-
The following information is available for two different types of businesses for the 2011 accounting period. Dixon Consulting is a service business that provides consulting services to small...
-
Marino Basket Company had a \(\$ 6,200\) beginning balance in its Merchandise Inventory account. The following information regarding Marino's purchases and sales of inventory during its 2011...
-
On March 6, 2011, Bob's Imports purchased merchandise from Watches Inc. with a list price of \(\$ 31,000\), terms \(2 / 10, n / 45\). On March 10, Bob's returned merchandise to Watches Inc. for...
-
The following events apply to Tops Gift Shop for 2012, its first year of operation: 1. Acquired \(\$ 45,000\) cash from the issue of common stock. 2. Issued common stock to Kayla Taylor, one of the...
-
Indicate whether each of the following costs is a product cost or a period (selling and administrative) cost. a. Transportation-in. b. Insurance on the office building. c. Office supplies. d. Costs...
-
The photoelectric effect is studied using a tungsten target. The work function of tungsten is 4.5 eV. The incident photons have energy 4.8 eV. (a) What is the threshold frequency? (b) What is the...
-
Identify the Critical Infrastructure Physical Protection System Plan.
-
There are eight different five-carbon alkyl groups. (a) Draw them. (b) Give them systematic names. (c) In each case, label the degree of substitution (primary, secondary, or tertiary) of the head...
-
Use a Newman projection, about the indicated bond, to draw the most stable conformer for each compound. (a) 3-methylpentane about the C2-C3 bond (b) 3, 3-dimethylhexane about the C3-C4 bond
-
(a) Draw the two chair conformations of cis-1, 3-dimethylcyclohexane and label all the positions as axial or equatorial. (b) Label the higher-energy conformation and the lower-energy conformation....
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


