Which of the two phenols in each set is more acidic? Explain. (a) 2,5-dinitrophenol or 2,4-dinitrophenol (b)
Question:
Which of the two phenols in each set is more acidic? Explain.
(a) 2,5-dinitrophenol or 2,4-dinitrophenol
(b) phenol or m-chlorophenol
(c)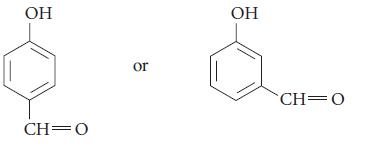
Transcribed Image Text:
OH CH 0 or OH CH=0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 24Dinitrophenol is more acidic because its conjugatebase anion has more important re...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the, two phenols in each set is more acidic? Explain. Phenol or m-chlorophenol
-
Maya earns $50/hour. John earns $45/hour + 5% commission + bonus (commission and bonus are based on his performance). (Note: Some economics concepts you may consider -- opportunity cost, law of...
-
Assume electricity is generated by three technologies: a base load technology with cost function C b (x b ) and proportional emission coefficient b ; gas turbines with cost function C g (x g ) and...
-
Show that the clock in S reads 100 min when the light flash reaches B, which is traveling away from C with speed 0.6c. y:V= 0.6c -100 cmin- A' Flashbulb
-
To finance your car, you have decided to take a car loan in the amount of $15,000 from your credit union. You will pay off this loan over 60 months. If the required monthly payment is $322.41, what...
-
Describe the authoritative leadership style. AppendixLO1
-
RadioShack Corporation primarily engages in the retail sale of consumer electronics goods and services through 4,467 company-operated stores under the RadioShack brand, located throughout the United...
-
Required information (The following information applies to the questions displayed below.) At the beginning of the year, Anna began a calendar-year business and placed in service the following assets...
-
The following compound, unlike most phenols, is soluble in neutral aqueous solution, but insoluble in aqueous base. Explain this unusual behavior. H -N(CH3)3 CI
-
Suggest a mechanism for the oxo reaction (Eq. 18.63) involving intermediates that are consistent with the 16- and 18-electron rules. H,C=CH2 + H2 + CO ethylene HCO (CO)4 100-120 C CHCHCH...
-
Stetson Corporation uses the equity method to account for its ownership of 30% of the common stock of Pike Packing. During 2017, Pike reported a net in- come of $80,000 and declares and pays cash...
-
Sams old friend Dot is considering setting up a business offering historical boating trips along the River Thames. Dot thinks that she may be able to make a good living out of this. She has carried...
-
Arrow Industries employs a standard cost system in which direct materials inventory is carried at standard cost. Arrow has established the following standards for the direct costs of one unit of...
-
Explain the financial effect (increase, decrease, or no effect) of each of the following transactions on stockholders' equity: a. Purchased supplies for cash. b. Paid an account payable. c. Paid...
-
What type of account-asset, liability, stockholders' equity, dividend, revenue, or expense-is each of the following accounts? Indicate whether a debit entry or a credit entry increases the balance of...
-
Is it possible for an accounting transaction to only affect the left side of the accounting equation and still leave the equation in balance? If so, provide an example.
-
An x-ray photon with wavelength 6.00 pm collides with a free electron initially at rest. What is the maximum possible kinetic energy acquired by the electron?
-
Write a paper about how diet relates to breast cancer in women study design to use: case control study purpose & rationale the purpose of this final project is to utilize the methods and...
-
The following names are all incorrect or incomplete, but they represent real structures. Draw each structure and name it correctly. (a) 2-ethylpentane (b) 3-isopropylhexane (c)...
-
Provide IUPAC names for the following compounds. (a) (CH3)2CHCH2CH3 (b) CH3-C(CH3)2-CH3 (c) (d) (e) (f) CH CH CHCH CH,CHCHCH le ' CH CH,CH, CH CH CH, CH CH C(CH CH,CH,CHCHCH, CH(CH2 CH CHCH,CH, CH)C...
-
In each pair of compounds, which compound has the higher boiling point? Explain your reasoning. (a) octane or 2,2,3-trimethylpentane (b) Nonane or 2-methylheptane (c) 2, 2, 5-trimethylhexane or nonane
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


