The following compound, unlike most phenols, is soluble in neutral aqueous solution, but insoluble in aqueous base.
Question:
The following compound, unlike most phenols, is soluble in neutral aqueous solution, but insoluble in aqueous base. Explain this unusual behavior.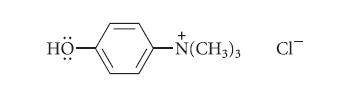
Transcribed Image Text:
HỌ -N(CH3)3 CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The solubility of most phenols in base due to their 1 charge when ionized Their c...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Toronto Tutors has been offering home tutoring services around the Toronto area since 2008. Roberta Draper started the business from her home, setting up tutoring sessions for math, science, and...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Consider again examples 9.2 and 9.3 , and once again assume that there are two states of the world for each random variable, denoted by V L j , V H j and n L , n H . Denote the probabilities for...
-
The time interval between the reception of the flashes at A and B in Problems 26 and 27 is 75 min according to the observer in S. How much time does he expect to have elapsed on the clock at A during...
-
What equal series of payments must be paid into a sinking fund in order to accumulate each given amount? (a) $11,000 in 10 years at 8% compounded semiannually when payments are semiannual. (b) $3,000...
-
Describe the coercive leadership style. AppendixLO1
-
List and describe the options available for the location of the information security functions within the organization. Discuss the advantages and disadvantages of each option.
-
Compute the IRR statistic for Project E. The appropriate cost of capital is 7 percent. Note: Do not round intermediate calculations and round your final answer to 2 decimal places. Project E Time: 0...
-
Given the structure of phenanthrene, draw structures of (a) 9,10-phenanthroquinone (b) 1,4-phenanthroquinone phenanthrene L 5 00 a N 10
-
Which of the two phenols in each set is more acidic? Explain. (a) 2,5-dinitrophenol or 2,4-dinitrophenol (b) phenol or m-chlorophenol (c) OH CH 0 or OH CH=0
-
Using the Web, research Mafia boys exploits. When and how did he compromise sites? How was he caught?
-
Brice Looney owns a small retail ice cream parlor. He is considering expanding the business and has identified two attractive alternatives. One involves purchasing a machine that would enable Mr....
-
A positively charged particle initially at rest on the ground moves \(4.0 \mathrm{~m}\) upward in \(2.00 \mathrm{~s}\). If the particle has a chargeto-mass ratio of \(10 \mu \mathrm{C} / \mathrm{g}\)...
-
Central States Telecom provides communication services in Iowa, Nebraska, the Dakotas, and Montana. Central States purchased goodwill as part of the acquisition of Sheldon Wireless Company, which had...
-
Shown below is selected information from the financial records of Merris Corporation as of December 31: Required a. Determine which of the above items will appear on the statement of cash flows and...
-
Pippa runs a photographic studio specializing in black and white portrait photography. Clients book a one hour studio session and are entitled to receive two large photographs of their choice from...
-
A photoelectric effect experiment is performed with tungsten. The work function for tungsten is 4.5 eV. (a) If ultraviolet light of wavelength 0.20 m is incident on the tungsten, calculate the...
-
A 2500-lbm car moving at 15 mi/h is accelerated at a constant rate of 15 ft/s 2 up to a speed of 50 mi/h. Calculate force and total time required?
-
Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match the description. (a) An isopropylheptane (b) A diethyldecane (c) A...
-
Give the IUPAC names of the following alkanes. (a) CH3C(CH3)2CH(CH)CH3)CH2CH2CH(CH3)2 (b) (c) (d) (e) (f) (g) (h) CH,CH CHCH CH, CH CH CH,CHCH CH,CHCH CH,CH CH,CH, CH, CH,CH, CH,CH,CH, C(CH,CH),...
-
Construct a graph, similar to Figure 3-11, of the torsional energy of 3-methylpentane along the C2-C3 bond. Place C2 in front, represented by three bonds coming together in a Y shape, and C3 in back,...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...

Study smarter with the SolutionInn App


