Outline a sequence of reactions by which d-glucose can be converted into methyl 2,3,4,6-tetra-O-acetyld-glucopyranoside. ACO ACOCH ACO-
Question:
Outline a sequence of reactions by which d-glucose can be converted into methyl 2,3,4,6-tetra-O-acetyld-glucopyranoside.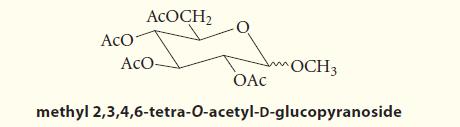
Transcribed Image Text:
ACO ACOCH₂ ACO- OCH 3 OAC methyl 2,3,4,6-tetra-O-acetyl-D-glucopyranoside
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
In solving problems of this sort in which apparently similar hydroxy groups are converte...View the full answer

Answered By

Moses mwangi
With prior writing experience, be sure that I will give a great grade, If not an A+, it will be something close to this. My reviews speaks it all, Try me!!
4.80+
78+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline a sequence of reactions that would bring about the conversion of aniline into each of the following compounds. (a) Benzyl alcohol (b) N-phenyl-2-butanamine
-
Outline a sequence of reactions that will bring about each of the following conversions. o-glucopyranose to 2,3,4,6-tetra-O-benzyl-o-glucopyranose
-
(A) Using Figure 21-19, write chemical equations for the sequence of reactions by which borax is converted to diborane. (B) Using Figure 21-19, write chemical equations for the sequence of reactions...
-
Given a sorted array of Comparable items, write functions floor () and ceiling () that return the index of the largest (or smallest) item not larger (or smaller) than an argument item in logarithmic...
-
The rest energy of a proton is about 938 MeV. If its kinetic energy is also 938 MeV, find (a) Its momentum (b) Its speed.
-
An aeration basin is used to oxygenate wastewater at 283 K. The basin is filled to a depth of 4.55 m with 283 m 3 wastewater of 0.050 mmole/L initial dissolved oxygen (O 2 ) concentration. The filled...
-
12-7. What are the three degrees of distribution density?
-
According to data from the U.S. Department of Energy, the average retail price of regular gasoline rose from $1.16 in 1990 to $2.52 in 2015, a 117% increase. a. Other things equal, describe the...
-
The debt created by a business when it borrows from a vendor or supplier is called a ( n ) : a . contingent liability. b . account payable. c . account receivable. d . intangible asset.
-
Name each of the following aldoses. In part (a), work back to the Fischer projection and consult Fig. 24.3. In part (b), decide which carbons have configurations different from those of glucose, and...
-
Convert the Fischer projection of -d-glucopyranose into a Haworth projection, a line-and-wedge structure, and a chair conformation. (A Haworth projection is defined below.)
-
Mr Norman set up a new business on 1 January 20X8. He invested 50,000 in the new business on that date. The following information is available. Required: (a) A purchases budget for each of the first...
-
The curved rod has a diameter \(d\). Determine the vertical displacement of end \(B\) of the rod. The rod is made of material having a modulus of elasticity of \(E\). Consider only bending strain...
-
If the inertial measurement system were written in C++ according to the design fragment described in Chapter 5, describe the testing strategy you would use. If possible, try to design some test cases.
-
Determine the displacement at point \(C\) of the W14 \(\times 26\) beam made from A992 steel. 8 kip A -5 ft 5 ft. B C -5 ft 5 ft- 8 kip D
-
The beam is subjected to the loading shown. Determine the slope at \(B\) and displacement at \(C\). \(E I\) is constant. Ta Mo C b B
-
A mass, connected to a damper as shown in Fig. 14.30, is subjected to a force \(F(t)\). Find the frequency-response function \(H(\omega)\) for the velocity of the mass. m F(t) y(1) FIGURE 14.30...
-
Why does a particle confined to a finite box have only a finite number of bound states?
-
What are the principal alloying elements in SAE 4340 steel?
-
Label the reactants in these acid-base reactions as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions. (a) (b) (c)...
-
Predict the products of the following acid-base reactions. (a) (b) (c) (d) (e) (f) (g) (h) CH,COOH + (CH, ),N : H2O + NH3 HCOOH + CH,O- NH,CH,COOH 2 OH
-
The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red. (a) Show the structure of the conjugate base of each acid, including any...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


