Noting the LX character of the allyl ligand in Table 18.1, sketch the allylmetal interaction, showing both
Question:
Noting the LX character of the allyl ligand in Table 18.1, sketch the allyl–metal interaction, showing both L-type and X-type bonds. Use M as a general metal.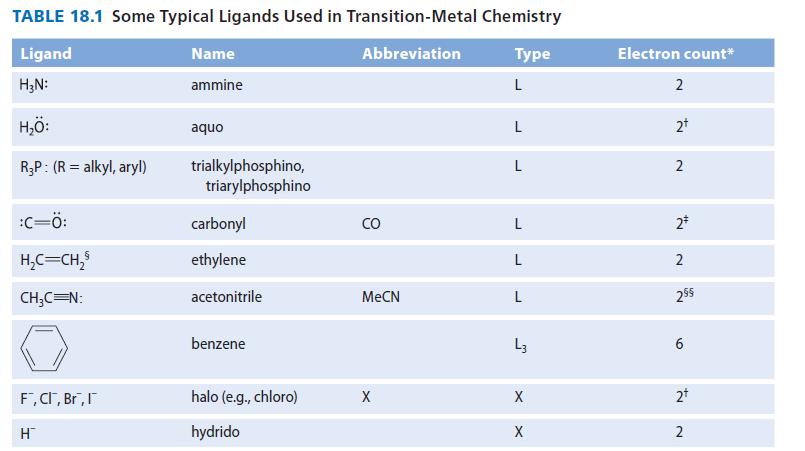
Transcribed Image Text:
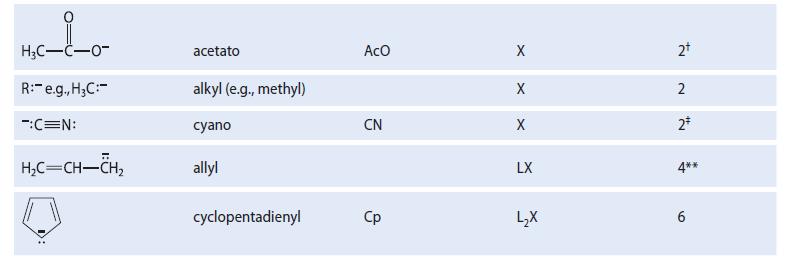
TABLE 18.1 Some Typical Ligands Used in Transition-Metal Chemistry Abbreviation Ligand H₂N: H₂O: R3P: (R = alkyl, aryl) :c=0: H₁₂C=CH₂5 CH₂C=N: F, Cl, Br, I H™ Name ammine aquo trialkylphosphino, triarylphosphino carbonyl ethylene acetonitrile benzene halo (e.g., chloro) hydrido со MeCN X Type L L L L L L L3 X X Electron count* 2 2+ 2 2* 2 295 6 2+ 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The bond to one allylic carbon is an Xtype bond That is if we break the me...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Emily Jackson (Social Security number 765-12-4326) and James Stewart (Social Security number 466-74-9932) are partners in a partnership that owns and operates a barber shop. The partnership's first...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Modify Lookup to make a program LookupAndPut that allows put operations to be specified on standard input. Use the convention that a plus sign indicates that the next two strings typed are the...
-
(a) In the reference frame of the muon in Problem 4, how far does the laboratory travel in a typical lifetime of 2 s? (b) What is this distance in the laboratorys frame?
-
You are considering two types of electric motors for your paint shop. Financial information and operating characteristics are summarized as follows: If you plan to operate the motor for 2,000 hours...
-
Describe ethically neutral leadership. AppendixLO1
-
"I know headquarters wants us to add that new product line," said Dell Havasi, manager of Billings Company's Office Products Division. "But I want to see the numbers before I make any move. Our...
-
Marshall-Miller & Company is considering the purchase of a new machine for $50,000, installed. The machine has a tax life of 5 years, and it can be depreciated according to the depreciation rates...
-
Which of the two compounds in each of the following sets should react more rapidly in a nucleophilic aromatic substitution reaction with CH 3 O in CH 3 OH? Explain your answers. (a) NO or F NO (b) NO...
-
Within each series, arrange the compounds according to increasing rates of their reactions by the S N 1E1 mechanism. Explain your reasoning. (b) Br -C=CH, A Cl -CH-CH3 A Br | -CH-CH3 B B Br C Cl...
-
In 2019, Hunter and Monda (both under age 50) had compensation income of $1,000,000 and $200,000, respectively. Adjusted gross income on their joint return was $1,200,000, and neither taxpayer was a...
-
The file NFL2012data.xlsx contains scores of all the NFL 2012 regular-season games. Rate the teams. Even though the Colts were 106, your ratings have the Colts as well below the average team. Can you...
-
A certain company reorders envelopes when its stock drops to 12 boxes, although demand for envelopes during lead time is normally distributed with a mean of 10 boxes and a standard deviation of 3...
-
Indicate the uses of budgeting and construct various budgets, including the cash budget, from relevant data.
-
Complete the double entry for each of the following transactions: a The owner of a business pays additional capital to the company; the cash account is debited and it is credited to the __________. b...
-
The file named Worldball.xlsx contains all the scores from the 2006 World Basketball Championships. Rate the teams. Who were the best three teams?
-
In gamma-ray astronomy, the existence of positrons (e+) can be inferred by a characteristic gamma ray that is emitted when a positron and an electron (e) annihilate. For simplicity, assume that the...
-
Write an essay describing the differing approaches of nursing leaders and managers to issues in practice. To complete this assignment, do the following: 1. Select an issue from the following list:...
-
(a) Using bond-dissociation enthalpies from Table 4-2 (page 143), calculate the heat of reaction for each step in the free-radical bromination of methane. (b) Calculate the overall heat of reaction....
-
The reaction of tert-butyl chloride with methanol Is found to follow the rate equation Rate = kf [(CH3)3C-Cl] (a) What is the kinetic order with respect to tert-butyl chloride? (b) What is the...
-
Under certain conditions, the bromination of cyclohexene follows an unusual rate law: Rate = kr [cyclo-hexene][Br2]2 (a) What is the kinetic order with respect to cyclo-hexene? (b) What is the...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


