Which of the two compounds in each of the following sets should react more rapidly in a
Question:
Which of the two compounds in each of the following sets should react more rapidly in a nucleophilic aromatic substitution reaction with CH3O¯ in CH3OH? Explain your answers.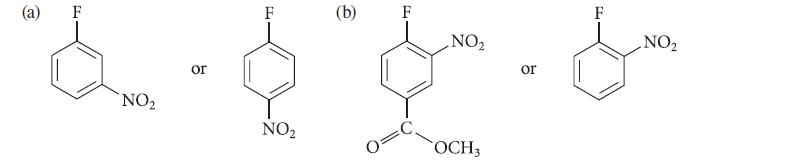
Transcribed Image Text:
(a) NO₂ or F NO₂ (b) NO₂ OCH3 or PL F NO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a The second compound pfluoronitrobenzene reacts mo...View the full answer

Answered By

Nishtha Goel
have more than 6 years of teaching experience in ICSE and CBSE School of Subject Maths, Science and English. Also I have done MSc Chemistry and MA English and a little experience of process Control and Instrumentation.
For a question to solve, you first have a basic knowlegde and Understanding of the subject which I will provide to you. Moreover to understand the topic discussion plays an important role, when we discuss a certain topic with someone many questions arise in our mind and to solve that questions, I am available for you.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain how you could distinguish between the two compounds in each of the following sets using only 13C NMR spectroscopy. Trans - 1,2-cy clohexanediami ne and trans - 1,4-cyclohexanediamine
-
Which of the two isomers in each of the following sets should have the greater basicity at the carbonyl oxygen? Explain. H,C CHCHC- OCH or HCC-o-CH CH CH
-
(a) The following resonance-stabilized ion can protonate to give two different constitutional isomers. Give their structures and give the curved-arrow notation for their formation. (b) One of the...
-
Consider a situation with two countries that have abatement cost functions : for j=L and j=H. The countries have identical damage functions D(E)= d.E.For each country the parameters s j are drawn...
-
Jay has been posted to a remote region of space to monitor traffic. Toward the end of a quiet shift, a spacecraft goes by, and he measures its length using a laser device, which reports a length of...
-
MG Cutting Systems is considering an investment project with the following parameters, where all cost and revenue figures are estimated in constant dollars: The project requires the purchase of a...
-
Describe hypocritical leadership. AppendixLO1
-
Choose three traditional firms within the same industry that sell similar products or services to consumers. Based on their public Web sites, compare and contrast how effective these companies are in...
-
Question 9 1.2 pts Let's suppose you (USA dealer) imported 10 BMW (7 series) from a German dealer on March 1, 2018 at 50,000 each, payable in 30 days. The exchange rate on March 1, 2018 was 1.14...
-
Calculate the oxidation state of the metal in each of the following complexes. (a) O Mn-O- permanganate (b) Pd(PPH3)4 tetrakis(triphenylphosphine) palladium (c) CpFe ferrocene
-
Noting the LX character of the allyl ligand in Table 18.1, sketch the allylmetal interaction, showing both L-type and X-type bonds. Use M as a general metal. TABLE 18.1 Some Typical Ligands Used in...
-
Ethyl alcohol (C2H5OH(g)) at 25C is burned in a steady-flow adiabatic combustion chamber with 90 percent excess air that also enters at 25C. Determine the adiabatic flame temperature of the products...
-
Obtain the phase trajectories for a system governed by the equation \[\ddot{x}+0.4 \dot{x}+0.8 x=0\] with the initial conditions \(x(0)=2\) and \(\dot{x}(0)=1\) using the method of isoclines.
-
Indicate whether each of the following accounts normally has a debit balance or a credit balance. a. Land b. Dividends c. Accounts Payable d. Unearned Revenue e. Consulting Revenue f. Salaries...
-
Indicate whether each of the following accounts normally has a debit or credit balance. a. Common Stock b. Retained Earnings c. Land d. Accounts Receivable e. Insurance Expense f. Cash g. Dividends...
-
Match each of the items in the left column with the LO5, 6 appropriate annual report component from the right column: 1. The company's total liabilities 2. The sources of cash during the period 3. An...
-
Allegra Company has sales of \($167,000\) and a bicak-even sales point of \($123,000\). Compute Allegra s margin of safety and its margin of safety ratio.
-
A surgeon is attempting to correct a detached retina by using a pulsed laser. (a) If the pulses last for 20.0 ms and if the output power of the laser is 0.500 W, how much energy is in each pulse? (b)...
-
Read the following description and Write a response of it. The discretion of public administrators can be decreased, but not altogether eliminated. Officials will use their discretion in any given...
-
For each reaction, estimate whether ÎSo for the reaction is positive, negative, or impossible to predict. a. (b) The formation of diacetone alcohol: c. heat C10H22--> n-decane C3H6 + C7H16...
-
(a) Propose a mechanism for the free-radical chlorination of ethane, (b) Calculate ÎHo for each step in this reaction. (c) Calculate the overall value of ÎHo for this reaction. hu CH3...
-
Draw Lewis structures for the following free radicals. (a) The ethyl radical, (b) The tert-butyl radical, (CH3)3C (c) The isopropyl radical (2-propyl radical) (d) The iodine atom CH3 CH2
-
Suppose First Fidelity Bank engaged in the following transactions: (Click the icon to view the transactions.) Journalize the 2018 and 2019 transactions on First Fidelity's books. Explanations are not...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...
-
Supply costs at Coulthard Corporation's chain of gyms are listed below: March April May June July August September October November Client-Visits 11,666 11,462 11,994 13,900 11,726 11, 212 12,006...

Study smarter with the SolutionInn App


