(a) The following resonance-stabilized ion can protonate to give two different constitutional isomers. Give their structures and...
Question:
(a) The following resonance-stabilized ion can protonate to give two different constitutional isomers. Give their structures and give the curved-arrow notation for their formation.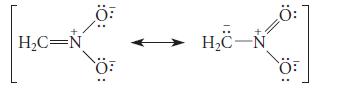
(b) One of the compounds in Problem 15.32 gives two different constitutional isomers as a product of its SN1 reaction with H2O. Which compound is it? Explain and give the structures of the two isomers.
Problem 15.32
The following isomers do not differ greatly in stability. Predict which one should react more rapidly in an SN1 solvolysis reaction in aqueous acetone. Explain.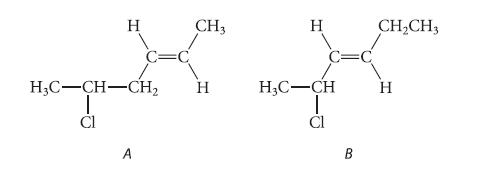
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





