The following isomers do not differ greatly in stability. Predict which one should react more rapidly in
Question:
The following isomers do not differ greatly in stability. Predict which one should react more rapidly in an SN1 solvolysis reaction in aqueous acetone. Explain.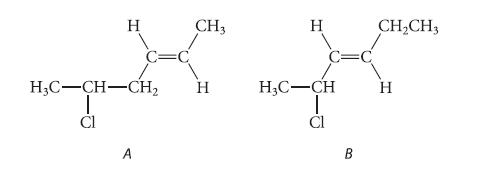
Transcribed Image Text:
H C=C A CH3 H3C-CH-CH₂ H Cl H H₂C-CH Cl B CH₂CH3 H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
According to Hammonds postulate Sec 48D of the text the rea...View the full answer

Answered By

Rukhsar Ansari
I am professional Chartered accountant and hold Master degree in commerce. Number crunching is my favorite thing. I have teaching experience of various subjects both online and offline. I am online tutor on various online platform.
5.00+
4+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) The following resonance-stabilized ion can protonate to give two different constitutional isomers. Give their structures and give the curved-arrow notation for their formation. (b) One of the...
-
Rank the following compounds in order of increasing reactivity (least reactive first) in an SN1 solvolysis reaction in aqueous acetone. Explain your answers. (The structure of tert-cumyl chloride is...
-
Why was the value of China's currency a dominant issue at the recent U.S. - China summit? Why is the United States pushing for a higher renminbi? Why is China reluctant to allow its currency to...
-
A firm only uses one input in its production function, labor (L = number of workers) to produce car rides (all the workers provide their own cars, gas, etc.). The firm's total revenue (TR) function...
-
In what order are assets listed on the balance sheet?
-
This is a more difficult but informative problem. James Brodrick & Sons, Inc. is growing rapidly and, if at all possible, would like to finance its growth without selling new equity. Selected...
-
Long-jump takeoff error. The long jump is a trackand- field event in which a competitor attempts to jump a maximum distance into a sandpit after a running start. At the edge of the pit is a takeoff...
-
A bank manager of City savings bank Inc. uses the managerial accounting system to track the costs of operating the various departments with in the bank. The departments include Cash Management,...
-
The Kirkland Department of Delta Manufacturing began the month of December with beginning Work in Process of 4,000 units that are 100% complete as to materials and 20% complete as to conversion...
-
Use a Frost circle to determine the -electron structure of (a) The cyclopentadienyl anion, which has a planar structure and six electrons; and (b) The cyclopropenyl cation, which has two electrons....
-
Suggest structures for the two constitutional isomers formed when 1,3-butadiene reacts with one equivalent of Br 2 . (Ignore any stereochemical issues.) Which of these products would predominate if...
-
Consider the salaries (in thousands of dollars) of a group of business executives: a. Construct a histogram of this data set. b. Describe the distribution shape. c. Based on the histogram, what...
-
Systems thinking is all about solving problemsin organizations, world situations, and even our personal lives. But it is not just a procedure; it is a different way of approaching problems. Our...
-
How would I display the following 3 principles in an entertaining infographic? Be very specific . Principle 1: Employee Engagement and Motivation Drawing from the Human Relations Movement theory and...
-
Shown below is a cross section of tubular member which is subjected to a torque T= 5.5 kN-m. It has a length L-3.0-m and the material shear modulus G=27 GPa. Dimensions: b=150 mm, h= 100 mm and t= 8...
-
The hip roof shown in the below Figure 2 is constructed of 2x10 rafters spaced 16 inches on center. The hip rafters are 1 -inch-wide by 12-inch-high GLBs. The roof has a slope of 4:12. Prepare a list...
-
2. Estimate the populations of Fargo, ND and Bismarck, ND in years of 2040 and 2050. Select a single value of population that you would use for design purposes in each year. You need to specify and...
-
In Problems 1-3, find the indicated derivative by using the rules that we have developed. 1. Dx(3x5) 2. Dx(x3 - 3x2 + x-2) 3. Dz(z3 + 4z2 + 2z)
-
Suppose that A is an m n matrix with linearly independent columns and the linear system LS(A, b) is consistent. Show that this system has a unique solution.
-
Propose structures for compounds that fit the following descriptions: (a) A hydrocarbon with seven lines in its 13C NMR spectrum (b) A six-carbon compound with only five lines in its 13C NMR spectrum...
-
Assign the resonances in the 13C NMR spectrum of methyl propanoate, CH3CH2CO2CH3(figure). TMS CH-cH 2 1 120 200 180 160 140 100 20 40 O ppm 60 Chemical shift (8) Intensity
-
Assign a chemical shift to each carbon in 6.methyl-5-hepten-2-ol(figure). (a) OH 0 ppm 200 180 160 140 120 100 80 60 40 20 Chemical shift (8) (b) 200 180 120 160 100 60 140 80 0 ppm Chemical shift...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


