Assign the resonances in the 13C NMR spectrum of methyl propanoate, CH3CH2CO2CH3(figure). TMS CH-cH 2 1 120
Question:
Assign the resonances in the 13C NMR spectrum of methyl propanoate, CH3CH2CO2CH3(figure).
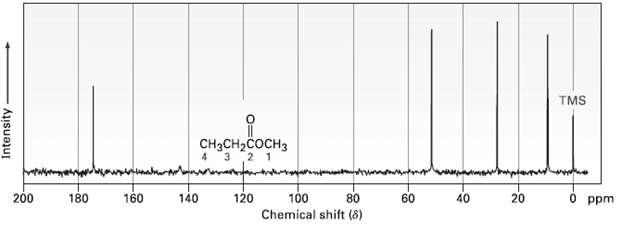
Transcribed Image Text:
TMS CнясH-cосHз 2 1 120 200 180 160 140 100 20 40 O ppm 60 Chemical shift (8) Intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Methyl propanoate has 4 unique carbons and each one absorbs in a specific region of the ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The standard 13C NMR spectrum of phenyl propanoate is shown here. Predict the appearance of the DEPT-90 and DEPT-135 spectra. 13C NMR 0-C-CH2CH3 pheny! propanoate 200 180 160140 10 100 80 40 20 0 8...
-
The mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectraldata. 100 80 60 40 20 aly 120 10 40 60 80 100 140 m/z TMS 200 180...
-
3-Methyl-2-butanol has five signals in its 13C NMR spectrum at 17.90, 18.15, 20.00, 35.05, and 72.75 ?. Why are the two methyl groups attached to C3 nonequivalent? Making a molecular model should be...
-
The preferred stock of Walter Industries Inc. currently sells for $36 a share and pays $2.50 in dividends annually. What is the firms cost of capital for the preferred stock?
-
In most models of entry deterrence, the incumbent engages in predatory practices that harm a potential entrant. Can these models be reversed, so that the entrant engages in predatory practices? If so...
-
Two neutral metal spheres on wood stands are touching. A negatively charged rod is held directly above the top of the left sphere, not quite touching it. While the rod is there, the right sphere is...
-
Using the data from Problem 11.2, determine which means are different using a = 0.05. AppendixLO1
-
Compare and contrast the four major types of marketing channels for consumer products. Through which type of channel is each of the following products most likely to be distributed? a. New...
-
Calculate the market price of a bond that promises a face value of $1,000 in 15 years. The bond pays semiannually an 8% annual coupon and the YTM is and annual rate of 9%.
-
The following selected ratios are available for Ice Inc.: Instructions a. Has the debt to total assets improved or weakened over the past three years? b. Has the interest coverage improved or...
-
Propose structures for compounds that fit the following descriptions: (a) A hydrocarbon with seven lines in its 13C NMR spectrum (b) A six-carbon compound with only five lines in its 13C NMR spectrum...
-
Assign a chemical shift to each carbon in 6.methyl-5-hepten-2-ol(figure). (a) OH 0 ppm 200 180 160 140 120 100 80 60 40 20 Chemical shift (8) (b) 200 180 120 160 100 60 140 80 0 ppm Chemical shift...
-
The following could relate to contract costing: (i) Work is for a period of long duration. (ii) Progress payments are amounts paid for the contract throughout the course of the contract. (iii)...
-
Imagine trying to manage and accommodate the needs of more than 185,000 people at once. Imagine a variety of voices, languages, cultures, ethnic backgrounds, families, lifestyles, ages, and...
-
Question 1 Case Study Cross-Boundary Teaming for Innovation: Integrating Research on Teams and Knowledge in Organizations In a growing number of cases, teams span organisational boundaries, not just...
-
Article Assignment Select a business article from a professional news source's business section or professional journal. The article selected is not to be opinion driven. Provide a personal opinion...
-
Research can be conducted for a variety of reasons including searching for effective change strategies that allow followers to perceive of someone as a leader rather than a manager or studying a...
-
The revenue recognition principle and the expense recognition principle require that the company recognize related revenue and expense transactions in the same accounting period. Discuss why this...
-
Examine the correlation matrix of independent variables and determine if multicollinearity is influencing your results. Correlation MatrixIndependent Variables X1 X2 X3 X4 X5 X6 X1 1 X2 .442** 1 2 X3...
-
Which of the following raises the credibility of areport? Which of the following raises the credibility of a report? Multiple Choice avoiding predictions avoiding the use of cause-effect statements...
-
Use the vant Hoff factors in Table 14.9 to calculate each colligative property: a. The melting point of a 0.100 m iron(III) chloride solution b. The osmotic pressure of a 0.085 M potassium sulfate...
-
Predict the esterification products of the following acid/alcohol pairs. (a) CH3CH2CH2COOH + CH3OH (b) CH3OH + HNO3 (c) 2 CH3CH2OH + H3PO4 (d) (e) COOH + CH,CH,OH
-
Both cis- and trans-2-methylcyclohexanol undergo dehydration in warm sulfuric acid to give 1-methylcyclohexene as the major alkene product. These alcohols can also be converted to alkenes by...
-
Show how you would convert (S)-hexan-2-ol to (a) (S)-2-chlorohexane (b) (R)-2-bromohexane (c) (R)-hexan-2-ol?
-
Each week you must submit an annotated bibliography. Entries of current events relating to the economic concepts and the impact on the company or the industry of your company. You must use acceptable...
-
Fluffy Toys Ltd produces stuffed toys and provided you with the following information for the month ended August 2020 Opening WIP Units 5,393 units Units Started and Completed 24,731 units Closing...
-
Part A Equipment 1,035,328 is incorrect Installation 44,672 is incorrect Anything boxed in red is incorrect sents 043/1 Question 9 View Policies Show Attempt History Current Attempt in Progress...

Study smarter with the SolutionInn App


