Use the vant Hoff factors in Table 14.9 to calculate each colligative property: a. The melting point
Question:
Use the van’t Hoff factors in Table 14.9 to calculate each colligative property:
a. The melting point of a 0.100 m iron(III) chloride solution
b. The osmotic pressure of a 0.085 M potassium sulfate solution at 298 K
c. The boiling point of a 1.22% by mass magnesium chloride solution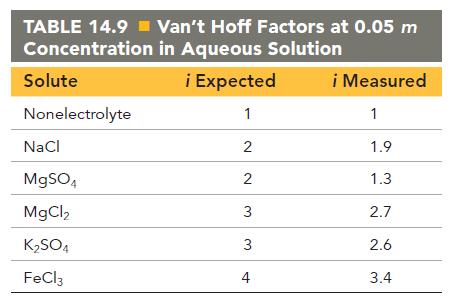
Transcribed Image Text:
TABLE 14.9 Van't Hoff Factors at 0.05 m Concentration in Aqueous Solution i Expected 1 Solute Nonelectrolyte NaCl MgSO4 MgCl, KSO4 FeCl3 2 2 3 3 4 i Measured 1 1.9 1.3 2.7 2.6 3.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
a 063...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the vant Hoff factors in Table 14.9, calculate the mass of solute required to make each aqueous solution: a. A sodium chloride solution containing 1.50 * 10 2 g of water that has a melting...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
How many orders of magnitude is 3 . 2 \ times 1 0 - 9 m smaller than 0 . 0 0 0 0 4 m ?
-
Jack Merritt is the controller for Universal Concrete Products (UCP), a manufacturing company with headquarters in Columbus, Ohio. UCP has seven concrete product plants located throughout the Midwest...
-
Stephanie Ling has been asked to conduct a breakeven analysis. In which category would she put the cost of rent for the manufacturing facility? a. Fixed cost b. Variable costc. Revenue cost d....
-
Explain the importance of ethics and values in effective management.
-
The notes that accompany a companys financial statements provide informative details that would clutter the amounts and descriptions presented in the statements. Refer to the financial statements of...
-
Required: a. Firm A has a margin of 11%, sales of $520,000, and ROI of 19%. Calculate the firm's average total assets. b. Firm B has net income of $76,000 turnover of 110, and average total assets of...
-
1. Recommend to the president that a meeting be arranged with the sales representatives entitled to a bonus and tell them that their checks are going to be delayed until Pugets financial picture...
-
A 1.2 m aqueous solution of an ionic compound with the formula MX 2 has a boiling point of 101.4 C. Calculate the vant Hoff factor (i) for MX 2 at this concentration.
-
Determine the required concentration (in percent by mass) for an aqueous ethylene glycol (C 2 H 6 O 2 ) solution to have a boiling point of 104.0 C.
-
Describe the extent to which any partnering makes sense for this project. What are the challenges and benefits to this partnering? What would prevent any further partnering?
-
Two scenarios about the future of the global economy in 2050 have emerged. Known as continued globalization, the first scenario is a (relatively) rosy one. Spearheaded by Goldman Sachs, whose...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Nike: Associating Athletes, Performance, and the Brand highlights Nike's use of marketing research...
-
Recall from Case 1.2 that Auto Concepts is a new division of a large automobile manufacturer that has been slowly losing market share to its competitors. Auto Concepts was created to reclaim the...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a diverging lens. \((b)\) Is the image real or virtual? (c) Is it upright or...
-
Show that the ray exiting the block in Figure P33.53 is parallel to the ray entering the block. Data from Figure P33.53
-
Niobium forms a substitutional solid solution with vanadium. Compute the weight percent of niobium that must be added to vanadium to yield an alloy that contains 1.55 1022 Nb atoms per cubic...
-
What key concerns must functional tactics address in marketing? Finance? POM? Personnel?
-
Light having a vacuum wavelength of 600 nm, traveling in a glass (n g = 1.50) block, is incident at 45 on a glassair interface. It is then totally internally reflected. Determine the distance into...
-
Derive an expression for the speed of the evanescent wave in the case of internal reflection. Write it in terms of c, n i , and i .
-
A large block of diamond is covered, on top, by a layer of water. A narrow beam of light travels upward in the solid and strikes the solidliquid interface. Determine the minimum incident angle that...
-
How much money should be deposited at age 50 in order to withdraw $30000 at the end of each year for 5 years if the first withdrawal is made at age 65. The account earns 8.25% compounded quarterly....
-
Suppose you are the money manager of a $4.98 million investment fund. The fund consists of four stocks with the following investments and betas: Stock Investment Beta A $ 240,000 1.50 B 700,000 (0.50...
-
Newton Company is privately owned by four individuals. The company sells athletic shoes, clothing, and accessories. An existing piece of equipment that keeps breaking down must be replaced....

Study smarter with the SolutionInn App


