Using the vant Hoff factors in Table 14.9, calculate the mass of solute required to make each
Question:
Using the van’t Hoff factors in Table 14.9, calculate the mass of solute required to make each aqueous solution:
a. A sodium chloride solution containing 1.50 * 102 g of water that has a melting point of -1.0 °C
b. 2.50 * 102 mL of a magnesium sulfate solution that has an osmotic pressure of 3.82 atm at 298 K
c. An iron(III) chloride solution containing 2.50 * 102 g of water that has a boiling point of 102 °C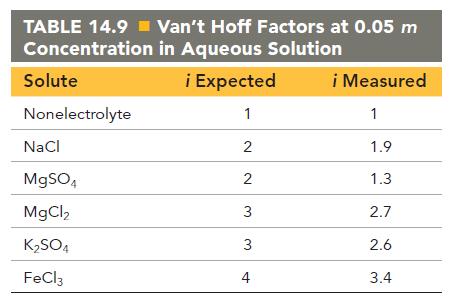
Transcribed Image Text:
TABLE 14.9=Van't Hoff Factors at 0.05 m Concentration in Aqueous Solution i Expected 1 Solute Nonelectrolyte NaCl MgSO4 MgCl, KSO4 FeCl3 2 2 3 3 4 i Measured 1 1.9 1.3 2.7 2.6 3.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Part a To calculate the mass of sodium chloride required to make a solution with a melting point of 10 Cwe can use the following equation T Kf m where ...View the full answer

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vant Hoff factors in Table 14.9 to calculate each colligative property: a. The melting point of a 0.100 m iron(III) chloride solution b. The osmotic pressure of a 0.085 M potassium sulfate...
-
were recorded The incomplete data in the table during an experiment in which two carts on a friction- less one-dimensional track collided head-on. What are the magnitudes of the average force F2...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
Danbury Inc is a resident Canadian corporation with worldwide operations. Canadian operations resulted in taxable income of $ 1 . 1 0 million and non - Canadian operations resulted in taxable income...
-
Carrie Ross is the Managing Partner of Ross, Sells, and Young, LLP, a mid-sized CPA firm. She has just finished reviewing the firms detailed income statement for the previous quarter. The statement...
-
A products packaging is used only to provide protection of the goods contents. True or False
-
Describe the concept of social responsibility and its role in todays organizations.
-
In five years, Kent Duncan will retire. He is exploring the possibility of opening a self-service car wash. The car wash could be managed in the free time he has available from his regular...
-
Use the financial statements supplied here for the International Motor Corporation (IMC) to answer the following questions: a. Calculate the cash conversion cycle for IMC for both 2015 and 2016. What...
-
Madrigal Corporation engaged in the following transactions during 2023: a. Madrigal spent $200,000 in an unsuccessful patent defense on October 1. As a result of the decision, the patent was...
-
A 1.2 m aqueous solution of an ionic compound with the formula MX 2 has a boiling point of 101.4 C. Calculate the vant Hoff factor (i) for MX 2 at this concentration.
-
Determine the required concentration (in percent by mass) for an aqueous ethylene glycol (C 2 H 6 O 2 ) solution to have a boiling point of 104.0 C.
-
Black Gold Petroleum Company is engaged in all phases of exploring, refining, and marketing of oil and petrochemical products. To ensure full compliance with all applicable laws, the company has a...
-
A simple experiment has long been used to demonstrate how negative pressure prevents water from being spilled out of an inverted glass. A glass that is fully filled by water and covered with a thin...
-
A golf ball is hit on a level fairway. When it lands, its velocity vector has rotated through an angle of 90. What was the launch angle of the golf ball? Pyo By Dyz =0 Uso Range R x max dya
-
Repeat Prob. 10-18 for signed-magnitude binary numbers. Prob. 10-18 Derive an algorithm in flowchart form for the comparison of two signed binary numbers when negative numbers are in signed-2's...
-
Tideview Home Health Care, Inc., has a bond issue outstanding with eight years remaining to maturity, a coupon rate of 10 percent with interest paid annually, and a par value of $1,000. The current...
-
Captain Billy Whirlywhirl Hamburgers issued 7%, 10-year bonds payable at 70 on December 31, 2010. At December 31, 2012, Captain Billy reported the bonds payable as follows: Captain Billy Whirlywhirl...
-
Silver and palladium both have the FCC crystal structure, and Pd forms a substitutional solid solution for all concentrations at room temperature. Compute the unit cell edge length for a 75 wt% Ag-25...
-
Coastal Refining Company operates a refinery with a distillation capacity of 12,000 barrels per day. As a new member of Coastal's management team, you have been given the task of developing a...
-
Figure P.4.91 shows a laserbeam incident on a wet piece of filter paper atop a sheet of glass whose index of refraction is to be measuredthe photograph shows the resulting light pattern. Explain what...
-
A large crystal of Fabulite is covered by a layer of carbon tetrachloride. A beam of light comes up through the crystal and impinges on the solidliquid interface. At what incident angle (at minimum)...
-
A beam of light from an argon laser ( 0 = 500 nm) traveling in a glass block (n g = 3/2) is totally internally reflected at the flat airglass interface. If the beam strikes the interface at 60.0 to...
-
A farmer is concerned that the price of wheat will drop by the time he is ready to sell his crop. He, therefore, enters into a futures contract on 5,000 bushels of wheat for 250 cents per bushel. The...
-
On December 1, ABC Company received $3,000 cash from a customer for 3 months of business services beginning December 1st. Prepare the journal entry to record the receipt of the 3,000 and the...
-
When Kevin started working 23 years ago, his salary was $59,349. His current salary is $159,408. When Kevin started working, the price level was 134, while the current price level is 157. What was...

Study smarter with the SolutionInn App


