The mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this
Question:
The mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectraldata.
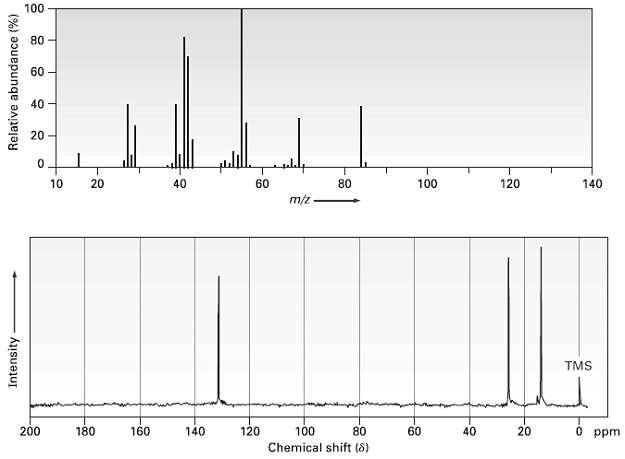
Transcribed Image Text:
100 80 60 40 20 aly 120 10 40 60 80 100 140 m/z TMS 200 180 160 120 100 80 60 20 0 ppm 140 40 Chemical shift (8) Intensity Relative abundance (%) 20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
The peak in the mass spectrum at mz 84 is probably the molecular ion of the unknown compound and co...View the full answer

Answered By

Rajat Gupta
used to take tution classes from my school time.
Conducted special topic claases during my graduation to help the students pass their exams.
Currently, teaching and conducting online claases during my post- graduation too.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The proton and 13C NMR spectra of a compound of formula C4H11N are shown here. Determine the structure of this amine, and give peak assignments for all of the protons in the structure. 200 180 160...
-
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown. (a) Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there? (b)...
-
The mass spectrum and infrared spectrum of an unknown compound are shown in Figures 13.27 and 13.28, respectively. Identify the compound. Figure 13.27 The mass spectrum for Problem 28. 100 E 80 3 60...
-
The Holtz Corporation acquired 80 percent of the 100,000 outstanding voting shares of Devine, Inc., for $7.20 per share on January 1, 2014. The remaining 20 percent of Devines shares also traded...
-
It is estimated that a firm contemplating entering the breakfast cereal market would need to invest $100 million to build a minimum efficient scale production plant (or about $10 million annually on...
-
The following scores represent the final examination grades for an elementary statistics course: Test the goodness of fit between the observed class frequencies and the corresponding expected...
-
Ms. V, a wealthy art collector in Country W, is interested in buying a rare painting from Mr. Y in Country Z. Both parties agree that the price is to be determined by an independent appraiser. V...
-
Brown Company (buyer) and Schmidt, Inc. (seller) engaged in the following transactions during February 2016: Brown Company DATE TRANSACTIONS 2016 Feb. 10 Purchased merchandise for $3,000 from...
-
The common stock of Air United had annual returns of 13.7 percent, 4.8 percent, -6.7 percent, and 27.9 percent over the last four years, respectively. What is the standard deviation of these returns?
-
Determine taxable income in each of the following independent cases. In all cases, the company was very profitable in all years prior to 2017 and it had retained earnings of $1,000,000 at the end of...
-
Propose structures for the three compounds whose 1H NMR spectra are shown. (a) C 5 H 10 O (b) C 7 H 7 Br (c) C 8 H 9 Br TMS O ppm 10 Chemical shift (8) TMS O ppm 10 9. 8. 3 2 Chemical shift (8) TMS O...
-
Compound A, a hydrocarbon with M + = 96 in its mass spectrum, has the 13 C spectral data that follow. On reaction with BH 3 followed by treatment with basic H 2 O 2 , A is converted into B, whose 13...
-
Explain the following terms: (a) The residual theory of dividends; (b) The clientele effect; (c) The signalling properties of dividends; (d) The bird in the hand argument.
-
Time ( s ) Velocity ( m / s ) 1 2 3 4 5 6 7 8 Calculate the velocity
-
The table below gives the data about Etruria's balance of payments. (All figures are in billions of dollars.) Foreign investment in Etruria Secondary (transfers) income received from abroad Primary...
-
Olive Corporation buys a material for P20 per unit. Sixteen thousand parts a year are needed. Carrying costs is P3.00 per unit and the ordering cost is P15. Required: Compute the economic order...
-
As a healthcare leader or manager, most of us are charged with supervising employees. The literature suggests the importance of hiring and retaining employees with high levels of emotional...
-
7-8. Evaluate the sum exactly. (10 points each) 7. 18 (1) n (33) "
-
Nutrix, Ltd., the maker of dietary supplements, has recently launched a new marketing campaign for Slender, an herbal supplement to increase weight loss. According to the product marketing materials,...
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
Potassium nitrate has a lattice energy of -163.8 kcal/mol and a heat of hydration of -155.5 kcal/mol. How much potassium nitrate has to dissolve in water to absorb 1.00 * 10 2 kJ of heat?
-
Show how Diels-Alder reactions might be used to synthesize the following compounds. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH 3COOCH CH3 CN CN CI CI Cl Cl Cl CI CI C chlordane CI CI Cl Cl CI C aldrin CN...
-
Furan and maleimide undergo a Diels-Alder reaction at 25 °C to give the endo isomer of the product. When the reaction takes place at 90 °C, however, the major product is the exo isomer....
-
(a) Sketch the pi molecular orbitals of hexa-1, 3, 5-triene (Figure 15-25). (b) Show the electronic configuration of the ground state of hexa-1, 3, 5-triene. (c) Show what product would result from...
-
Reporting bonds at fair value LO 1 4 6 [ Note: This is a variation of E 1 4 1 3 modified to consider the fair value option for reporting liabilities. ] Federal Semiconductors issued 1 1 % bonds,...
-
Define the purpose, goals and objectives of the mortgage broking business in which you are working, with your team members:
-
What is the risk profile of your company? (How much overall risk is there in this firm? Where is this risk coming from (market, firm, industry or currency)? (APPLE COMPANY LATEST DATA) How is the...

Study smarter with the SolutionInn App


