Within each series, arrange the compounds according to increasing rates of their reactions by the S N
Question:
Within each series, arrange the compounds according to increasing rates of their reactions by the SN1–E1 mechanism. Explain your reasoning.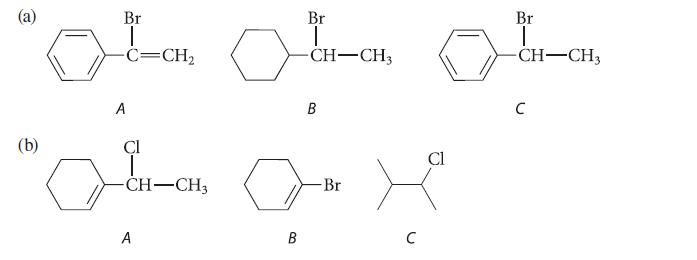
Transcribed Image Text:
(b) Br -C=CH, A Cl امي مسلم -CH-CH3 A Br | -CH-CH3 B B Br لى C Cl Br -CH-CH3 C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a b The o...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Within series, arrange the compounds according to increasing rates of their reactions by the SN1 - E1 mechanism. Explain your reasoning. CH CH Br
-
Within each series arrange the compounds in order of increasing stability: C(CH3)3 HO CH,CH(CH)
-
Within set, rank the compounds in order of increasing rates of their SN2 reactions. Explain your reasoning. 1-bromocyclohexene, bromocyclohexane, 1-(bromomethyl)cyclohexene
-
The example images in the text for Fade do not quite line up in the vertical direction (the mandrill's mouth is much lower than Darwin's). Modify Fade to add a transformation in the vertical...
-
The proper mean lifetime of a muon is 2 s. Muons in a beam are traveling at 0.999c. (a) What is their mean lifetime as measured in the laboratory? (b) How far do they travel, on average, before they...
-
Show that l is an eigenvalue of A and find one eigenvector corresponding to this eigenvalue. 2 A = 2 = -2 -1
-
Describe ethical leadership. AppendixLO1
-
Mr. Brooks is employed as a financial analyst by a large Canadian public firm located in Winnipeg. During 2018, his basic gross salary amounts to $63,000. In addition, he was awarded an $11,000 bonus...
-
1. Boundary Value Problem: A boundary value problem is outlined in the diagram below. Y3 Bico B2> B3 B3
-
Noting the LX character of the allyl ligand in Table 18.1, sketch the allylmetal interaction, showing both L-type and X-type bonds. Use M as a general metal. TABLE 18.1 Some Typical Ligands Used in...
-
Within each set, rank the compounds in order of increasing rates of their S N 2 reactions. Explain your reasoning. (a) Benzyl bromide, (3bromopropyl) benzene, pbromotoluene (b) 1bromocyclohexene,...
-
The chemical name for aspirin is acetylsalicylic acid. It is believed that the analgesic and other desirable properties of aspirin are due not to the aspirin itself but rather to the simpler compound...
-
Will the amount of an accrual always be an exact known amount, or could it be an estimate?
-
The reorder point for SKU 303 is 102 units, while average demand during the lead time on an order for SKU 303 is 97 units. How much safety stock is implied by SKU 303's reorder point policy?
-
Find the volume of the solid obtained by rotating the region bounded by the given curves about the specified line. Sketch the region, the solid and a typical disk or washer. -2x 3. y = ex, y = 0, x =...
-
2. Given the list of scores: Score1 = [ 10, 40, 50, 54, 55, 59, 63, 65, 70, 71, 75, 77, 79, 80, 99] The one-sample T-test is used to test whether the mean of Score1 is statistically different from...
-
Find the area of the triangle having the given measurements. Round to the nearest square unit. 13) C=100, a 3 yards, b = 8 yards Use Heron's formula to find the area of the triangle. Round to the...
-
A muon and an antimuon, each with a mass that is 207 times greater than an electron, were at rest when they annihilated and produced two photons of equal energy. What is the wavelength of each of the...
-
Continuation of Exercise 4-83. (a) What is the probability that the first major crack occurs between 12 and 15 miles of the start of inspection? (b) What is the probability that there are no major...
-
When a small piece of platinum is added to a mixture of ethene and hydrogen, the following reaction occurs: Doubling the concentration of hydrogen has no effect on the reaction rate. Doubling the...
-
(a) Draw the reaction-energy diagram for the reverse reaction: CH3 + HCl CH4 + Cl (b) What is the activation energy for this reverse reaction? (c) What is the heat of reaction (H) for this reverse...
-
Draw a reaction-energy diagram for the following reaction: CH3 + Cl2 CH3Cl + Cl The activation energy is 4 kJ mol (1 kcal mol), and the overall Ho for the reaction is -109kJ/mole (-26 kcal/mol) (b)...
-
Justice Corporation Comparative Balance Sheet December 31, 2025 and 2024 2025 2024 Assets Current Assets: $ Cash and Cash Equivalents 2,254 $ 1,876 Justice Corporation reported the following...
-
The Fields Company has two manufacturing departments forming and painting. The company uses the FIFO method of process costing at the beginning of the month the forming department has 33.000 units in...
-
A comparative balance sheet for Lomax Company containing data for the last two years is as follows: Lomax Company Comparative Balance Sheet This Year Last Year $ 96,000 $ 70,000 640,000 672,500...

Study smarter with the SolutionInn App


