Within each series arrange the compounds in order of increasing stability: C(CH3)3 HO CH,CH(CH)
Question:
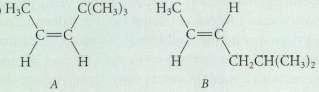
Transcribed Image Text:
C(CH3)3 HO CH,CH(CH)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Compound B is more stable than compound A because com...View the full answer

Answered By

Ashish Jaiswal
I have completed B.Sc in mathematics and Master in Computer Science.
4.90+
20+ Reviews
39+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In the following set, the NMR spectra of the compounds shown consist of a single resonance. Arrange the compounds in order of increasing chemical shift, smallest first. CH,CI, CH212 CH31
-
Arrange the compounds in order of increasing C---N bond length. Explain your answers. , NH, . NH> HN=CH2
-
In the following set, arrange the compounds in order of decreasing pKa, and explain your reasoning. 0 OCH, OCH3
-
A trader has made a sale of Rs.75,500 out of which cash sales amounted to Rs.25,500. He showed trade receivables on 31-3-2014 at Rs.25,500. Which concept is followed by him? a) Going concern b) Cost...
-
Identify one organization you, if you were/are an accounting professional, would like to join. Be sure to identify two resources that you would receive as a member and why you would find those...
-
Suppose you have just started a business to manufacture your newest invention, the photon gismo. Let's say you believe that after a few years on the market, photon gismos will sell for about...
-
Drip irrigation. In agriculture, water is distributed to the active root zone of plants using a drip irrigation system. The system involves drippers and drip lines. The quality of the water flow...
-
A company is forecasting the purchase of inventory from an overseas vendor with payment to be made in a foreign currency (FC). Assume an option was used as a hedging instrument for this forecasted...
-
Question 2 of 10 - / 10 III View Policies Current Attempt in Progress The following information is available for Sheffield Corp. for 2022. $ 148,629 67.362 69,216 1.434,687 revenue 2.478.180 305.910...
-
The AGRI Venture: An Integrated Marketing Communications Program. Chapter 16 states that there are three major forms of cooperative advertising: horizontal, ingredient-sponsored and vertical. Discuss...
-
(a) If the standard enthalpy change for the reaction 2-ethyl- I -butene I -hexene is + I 5.3 kJ mol-r (+3.66 kcal mol-t). and if Afli for l-hexere is -40.5 kJ mol-r (-9.68 kcal mol-1 2-methy...
-
Give the structure for each of the following: (a) 2-methylpropene (b) 5 -(3 -pentenyl)- 1, 3,6,8-decatetraene
-
Create several guidelines for developing good documentation.
-
Winston Electronics reported the following information at its annual meetings. The company had cash and marketable securities worth $1,235,740, accounts payables worth $4,160,391, inventory of...
-
Hooray Company has been manufacturing 12,000 units of Part A which is used to manufacture one of its products. At this level of production, the cost per unit is as follows: Direct materials P 4.80...
-
At the beginning of the period, the Grinding Department budgeted direct labor of $171,200 and property tax of $57,000 for 10,700 hours of production. The department actually completed 12,800 hours of...
-
The following information is available for Shamrock Corporation for the year ended December 31, 2025. Beginning cash balance $ 58,500 Accounts payable decrease 4,810 Depreciation expense 210,600...
-
In today's stock market, compounding is the key to making money in the future for one's investments. However, with decentralized currency growing rapidly (Crypto), how can one rely on TVM for FV...
-
Find the mean and median for the grouped data in the following table. Graduating Class Grade-Point Averages Interval 1.95-2.15 2.15-2.35 2.35-2.55 2.55-2.75 2.75-2.95 2.95-3.15 3.15-3.35 3.35-3.55...
-
2.) Find the Laplace transform of f(t) 7e-St cos 2t +9 sinh2 2t. Use Laplace Table. %3D
-
Give the structure of the alkene that could be used as a starting material to form chlorohydrin B in Study Problem 5.1. STUDY PROBLEM 5.1 Which of the following chlorohydrins could be formed by...
-
Alkene X of unknown structure gives the following products after treatment with ozone followed by aqueous H 2 O 2 : What is the structure of X? =0 cyclopentanone and HCCH,CH3 propionic acid
-
Give the products, and the mechanisms for their formation, when 2-methyl-1-hexene reacts with each of the following reagents. (a) Br 2 (b) Br 2 in H 2 O (c) Iodine azide (IN 3 )
-
Palisade Creek Co. is a merchandising business that uses the perpetual inventory system. The account balances for Palisade Creek Co. as of May 1, 2019 (unless otherwise indicated), are as follows:...
-
1-When accounting for an acquisition, goodwill is the difference between what two things? 2- What factors should be considered when deciding whether an acquisition should be financed with cash or...
-
What is the main friction Fluidity aims to address? REAL STATE

Study smarter with the SolutionInn App


