Provide two different reaction sequences that could be used to synthesize 4-methoxy-3-methylbiphenyl. Both sequences, however, should start
Question:
Provide two different reaction sequences that could be used to synthesize 4-methoxy-3´-methylbiphenyl. Both sequences, however, should start with both p-bromoanisole and m-bromotoluene.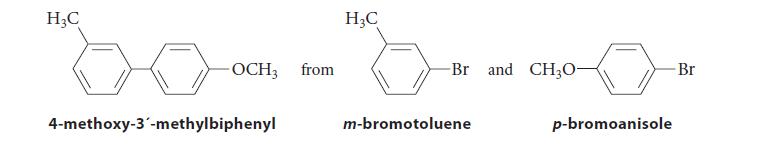
Transcribed Image Text:
H₂C -OCH3 from 4-methoxy-3'-methylbiphenyl H₂C -Br and CH3O- m-bromotoluene p-bromoanisole Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
The starting materials for a Suzuki coupling can be determined by mentally splitting the arylar...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a literature review for your study. See below for an example of a literature review. Your literature review should provide both analysis and synthesis of previous studies as related to the...
-
In 2015, John decided to start up his own brewery, Tasmania Microbrewery Inc (TMI). His family supported his decision and joined him in investing in the business. TMI began operations on January 1,...
-
In 2009, David Stott quit his job at a large beer company to start his own brewery, Arizona Microbrewery, Inc. (AMI). His family supported his decision and invested in the business along with David....
-
Use a software package such as Matlab or Mathematica to program the example described in section 7.3. (a) Assume the environmental regulator ignores the impact on the labor market and sets an...
-
A spaceship of proper length L = 400 m moves past a transmitting station at a speed of 0.76c. At the instant that the nose of the ship passes the transmitter, clocks at the transmitter and in the...
-
Consider the following set of investment projects, all of which have a three-year investment life: (a) Compute the net present worth of each project at i = 10%. Project Cash Flows A B D -$1,200...
-
Describe the emotional intelligence competency called self-awareness. AppendixLO1
-
1. Is this company running afoul of the Fair Labor Standards Act (FLSA)? Refer back to the discussion earlier in this chapter. Would this company be able to document that the store managers are...
-
Question 1 Molly Ltd bought 100% of the shares in Sam Ltd on 1 July 2020. Molly Ltd sold inventories to Sam Ltd on 1 May 2024 for $23,000 cash. Molly originally purchased the inventory for $14,000....
-
Draw a curved-arrow mechanism for the last (acid-catalyzed hydrolysis) step of Eq. 18.50. MgBr OCH3 + B(OCH3)3 B(OCH3)3 MgBr OCH3 H0 HO B(OH)2 OCH3 + 3 CH3OH 2+ + Mg+ + Br (18.50)
-
What product is expected when cyclopentene reacts with iodobenzene in the presence of triethylamine and a Pd(0) catalyst?
-
Dunbar Manufacturing PLC is a multi-product manufacturing company with four factories around the country. The auditors, Flossy & Co. LLP, have completed the interim audit for the year ending 31...
-
Given forecast errors of 4, 8, and -3, what is the MAD? What is the MSE?
-
Padgett Rentals can purchase a van that costs \($48,000\) ; it has an expected useful life of three years and no salvage value. Padgett uses straight-line depreciation. Expected revenue is...
-
Rainwater Corp. expects to sell 600 umbrellas in May and 400 in June. Each umbrella sells for \($15\). Rainwaters beginning and ending finished goods inventories for May are 75 and 50 units,...
-
Don Moon is the owner of ABC Cleaning. At the beginning of the year, Moon had \(\$ 2,400\) in inventory. During the year, Moon purchased inventory that cost \(\$ 13,000\). At the end of the year,...
-
Agua Ole is a distributor of bottled water. For each of items a through c, compute the amount of cash receipts or payments Agua Ol will budget for September. The solution to one item may depend on...
-
The minimum energy required to remove an electron from a metal is 2.60 eV. What is the longest wavelength photon that can eject an electron from this metal?
-
It is possible to investigate the thermo chemical properties of hydrocarbons with molecular modeling methods. (a) Use electronic structure software to predict cHo values for the alkanes methane...
-
(a) Write the propagation steps leading to the formation of dichloromethane (CH2Cl2) from chloromethane. (b) Explain why free-radical halogenations usually gives mixtures of products. (c) How could...
-
Draw resonance forms to show how the BHA radical is stabilized by delocalization of the radical electron over other atoms in the molecule.
-
The triphenylmethyl cation is so stable that some of its salts can be stored for months. Explain why this cation is so stable. triphenylmethyl cation
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


