Compound A is a by-product of the autoxidation of cumene, and compound B is a by-product of
Question:
Compound A is a by-product of the autoxidation of cumene, and compound B is a by-product of the acid-catalyzed conversion of cumene hydroperoxide to phenol and acetone.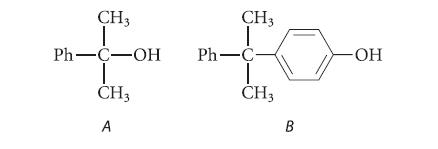
Draw a curved-arrow mechanism that shows how compound A can react with phenol under the conditions of Eq. 18.102 to give compound B.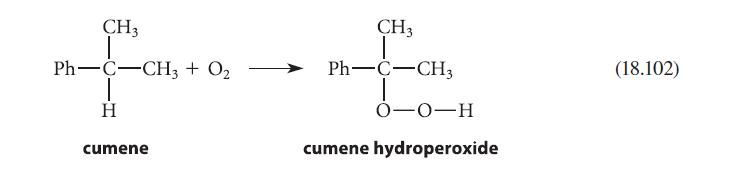
Transcribed Image Text:
CH3 Ph-C-OH T CH3 A CH3 Ph-C CH3 B -ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The alcohol A can form a relatively stable tertiary benzylic ca...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a stepwise mechanism for the hydrolysis of chlorobenzene under the conditions shown in Table 24.3. TABLE 24.3 Industrial Syntheses of Phenol Reaction and comments Chemical equation Reaction of...
-
Draw the structure of each of the following. (Some parts may have more than one correct answer.) (a) A nine-carbon ether that can be prepared by the Williamson synthesis. (b) An ether that would...
-
In the conversion shown in Fig. P18.77, the Diels-Alder reaction is used to trap a very interesting intermediate by its reaction with anthracene. From the structure of the product, deduce the...
-
Modify the test client in Turtle to take an odd integer \(n\) as a command-line argument and draw a star with \(\mathrm{n}\) points.
-
Herb and Randy are twin jazz musicians who perform as a trombonesaxophone duo. At the age of twenty, however, Randy got an irresistible offer to join a road trip to perform on a star 15 light-years...
-
Table 10.14 shows results when subjects were asked Do you think a person has the right to end his or her own life if this person has an incurable disease? and When a person has a disease that cannot...
-
Understand some of the ethical challenges that you may face as a project leader or project team member. AppendixLO1
-
Develop a multiple-regression model for auto sales as a function of population and household income from the following data for 10 metropolitan areas: a. Estimate values for bt). b, and h: for the...
-
On 30 June 2019, Stephen Ltd gained control of Hawking Ltd by purchasing all its share capital. On the control date, the fair values of Hawking Ltds following assets differed from their carrying...
-
Give the product(s) (if any) expected when p-iodotoluene or other compound indicated is subjected to each of the following conditions. (a) CHOH, 25 C (b) CH3O- in CHOH, 25 C (c) CHO, pressure, heat...
-
(a) Give the structure of the product formed in the reaction of urushiol with K 2 CO 3 and a large excess of methyl iodide. (b) Would this compound be likely to provoke the same allergic skin...
-
We repeat the information from exercise. a. Decide, at the 10% significance level, whether the data provide sufficient evidence to conclude that x is useful for predicting y. b. Find a 90% confidence...
-
n1 = 20, n2 = 25, S = 607, H1: 1 2. In Exercises 710, compute S, S, and the value of the test statistic z. Then find the P-value for the specified alternate hypothesis and values of n1, n2, and S.
-
To determine whether traffic levels differ between the morning and evening rush hours, a traffic engineer counted the number of cars passing through a certain intersection during five-minute periods...
-
Macon Timber Company established a \(\$ 150\) petty cash fund on January 1, 2012. Required a. Is the establishment of the petty cash fund an asset source, use, or exchange transaction? b. Record the...
-
Following is a bank reconciliation for Holt's Sandwich Shop for May 31, 2012: Because of limited funds, Holt's employed only one accountant who was responsible for receiving cash, recording receipts...
-
For each of the following situations, fill in the blank with FIFO, LIFO, or weighted average. a. b. c. d. e. f. would produce the highest amount of net income in an inflationary environment. would...
-
Suppose that you have a glass tube filled with atomic hydrogen gas (H, not H2). Assume that the atoms start out in their ground states. You illuminate the gas with monochromatic light of various...
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
1. Draw the important resonance forms for each compound. 2. Label the hybridization and bond angles around each atom other than hydrogen. 3. Use a three-dimensional drawing to show where the...
-
List each set of compounds in order of increasing boiling point. (a) Hexane, octane, and decane (b) Octane, (CH3)3 C-C(CH3)3 and CH3CH2C(CH3)2CH2CH2CH3
-
Draw a graph, similar to Figure 3-11, of the torsional energy of 2-methylbutane as it rotates about the C2¬C3 bond. Figure 3-11 3.8 kJ (0.9 kcal) 15 kJ 21 kJ (3.6 kcal) (5 kcal) -21 kJ (5 kcal)...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


