Question: Complete the reactions given in Fig. P17.45 by proposing structures for the major organic products. (a) HC=CH-CH=CH-CHMgBr + DO (b) + HCH HC=CH-CH=CH-CHMgBr + HC-CH
Complete the reactions given in Fig. P17.45 by proposing structures for the major organic products.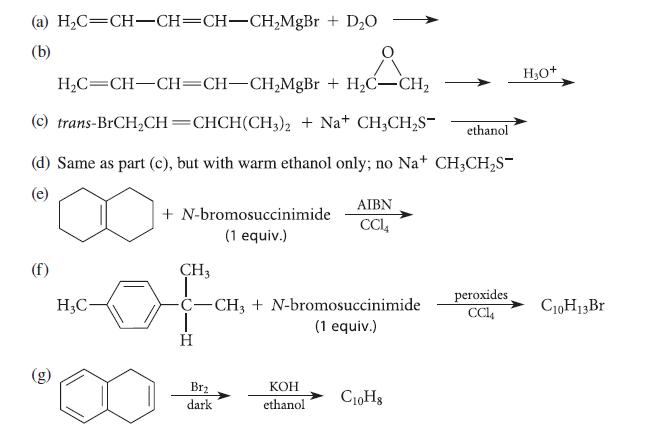
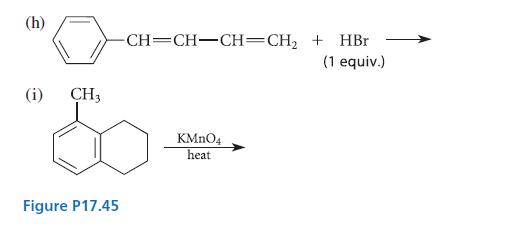
(a) HC=CH-CH=CH-CHMgBr + DO (b) + HCH HC=CH-CH=CH-CHMgBr + HC-CH (c) trans-BrCHCH=CHCH(CH3)2 + Na+ CH3CHS- (d) Same as part (c), but with warm ethanol only; no Na+ CH3CHS- (e) (f) HC- + N-bromosuccinimide (1 equiv.) CH3 | C-CH3 + N-bromosuccinimide (1 equiv.) T H Brz dark AIBN CCL4 KOH ethanol ethanol C10H8 peroxides CCl4 H3O+ C10H13 Br
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
b Two allylic Grignard reagents are in equilibrium and each reacts with DO HCCHCHCHCHMgBr HCCHCHCHCH DO HCCHCHCHCHD MgBr DO HC CHCHCHCH D Note in this ... View full answer

Get step-by-step solutions from verified subject matter experts


