Propose a curved-arrow mechanism for each of the reactions given in Fig. P17.46. (b) CH3(CH)3 C=C-CH-Br +
Question:
Propose a curved-arrow mechanism for each of the reactions given in Fig. P17.46.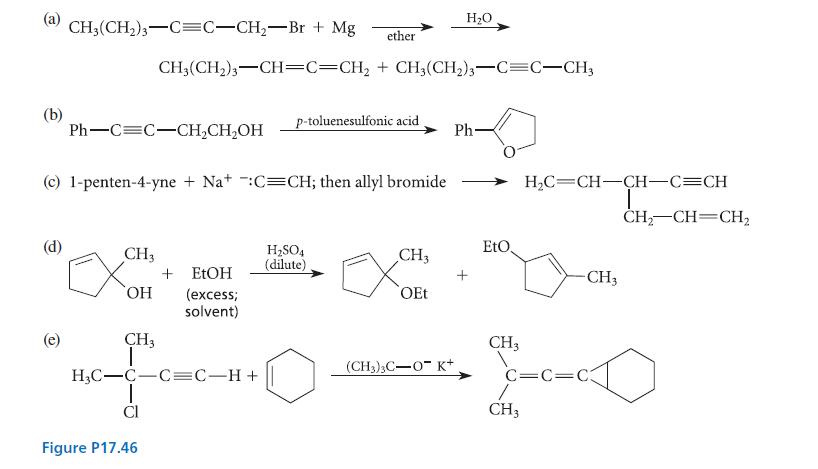
Transcribed Image Text:
(b) CH3(CH₂)3 C=C-CH₂-Br + Mg (e) Ph-C=C-CH₂CH₂OH CH3 (c) 1-penten-4-yne + Na+ :C=CH; then allyl bromide + EtOH OH (excess; solvent) CH3 CH₂(CH₂)3-CH=C=CH₂ + CH3(CH2)3-C=C-CH3 H₂C-C-C=C-H+ Cl Figure P17.46 ether p-toluenesulfonic acid. H₂SO4 (dilute) CH3 OEt H₂O (CH3)3C-OK+ Ph- EtO. CH3 H₂C CH-CH-C=CH L CH3 -CH3 C=C=C3 CH₂ CH CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Propargylic Grignard reagents like allylic Grignard reagents are an equilibrium mixture of two con...View the full answer

Answered By

Niala Orodi
I am a competent and an experienced writer with impeccable research and analytical skills. I am capable of producing quality content promptly. My core specialty includes health and medical sciences, but I can competently handle a vast majority of disciplines.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The total amount paid in by__________for the shares they purchase is described as common stock. Select answer from the options below creditors bondholders employees stockholders
-
Give a curved-arrow mechanism for each of the reactions given in Fig. P20.51. OC,H, dil. HCI (catal ,- OC2Hs an orthoester 0 CIH O C CH dil HOI (catalyst) CH OH (e) H,C ,--, carbon monoxide CH Ph Ph...
-
Give a curved-arrow mechanism for each of the reactions given in Fig. P20.52. (a) (b) OEt 1 H3C-C-OEt + HO T OEt an orthoester (c) HC H3C C-CH3 COH dil. HCl (catalyst) CH3OH + C=CH + :C=O: dil. HCI...
-
Modify BST to add a method rangeSearch () that takes two keys as arguments and returns an iterable over all keys that are between the two given keys. The running time should be proportional to the...
-
An interstellar spaceship travels from the earth to a distant star system 12 light-years away (as measured in the earths frame). The trip takes 15 years as measured on the ship. (a) What is the speed...
-
Chittenden County had the following federal award activity during the most recent fiscal year: Required a. Based on size, which programs would be considered Type A programs? Type B programs? b. You...
-
In how many ways can four of the numbers be selected if order is important?
-
Ralphs Bow Works (RBW) is planning to add a new line of bow ties that will require the acquisition of a new knitting and tying machine. The machine will cost $1,000,000. It is classified as a 7-year...
-
The underlying asset price follows a Black-Scholes model with initial price S0 = 10 euro, volatility = 20% per year, risk free rate r = 2% per year. You sell an option that pays 100 if S 2 1 100 and...
-
Specify the relationship(s) of the compounds in each of the following sets. Choose among the following terms: identical compounds, epimers, anomers, enantiomers, diastereomers, constitutional...
-
Consider the relative rates of the two solvolysis reactions in acetic acid solvent shown in Fig. P17.50. (a) Suggest a reason that compound A undergoes solvolysis much faster than compound B. (b)...
-
Let (X, Y, Z) be uniformly distributed on a three-dimensional box with side lengths 3, 4, and 5. Find P(X < Y < Z).
-
The adjusted trial balance columns of a worksheet for Levitt Corporation are shown below. The worksheet is prepared for the year ended December 31, Complete the worksheet by (a) entering the adjusted...
-
Derive the commutator $\left[Q_{i}, Q_{j} ight]=i \epsilon_{i j k} Q_{k}$ for the charge defined in Eq. (33.4). Use the charge (33.4) to write the commutator, displaying explicit matrix indices...
-
Verify that the potential $V(\pi, \sigma)$ can be written as Eq. (33.11), and that if $\epsilon=0$ and the symmetry is implemented in the Wigner mode the masses for the $\pi$ and $\sigma$ fields are...
-
Figure 5.7 shows a number of yield curves at various points in time. Go to www.treasury.gov, and in the Resource Center at the top of the page click on Data and Charts Center. Find the Treasury yield...
-
The number of vacation days used by a sample of 20 employees in a recent year In Exercises 2326, use technology to draw a box-and-whisker plot that represents the data set. 3 9 2 17 5 3 2 2 6 4 0 10...
-
Electrons are accelerated through a potential difference of 38.0 V. The beam of electrons then passes through a single slit. The width of the central fringe of a diffraction pattern formed on a...
-
Presented below are income statements prepared on a LIFO and FIFO basis for Kenseth Company, which started operations on January 1, 2024. The company presently uses the LIFO method of pricing its...
-
Without drawing the MOs, state whether the 7r-molecular orbital 6 in 1,3,5,7,9-decapentaene (a 10-carbon conjugated alkene) is symmetric or anti symmetric with respect to the reference plane; is...
-
What do the pericyclic selection rules have to say about the position of equilibrium in each of the reactions given in Fig. P27.30? Which side of each equilibrium is favored and why? Fig. P27.30 (a)...
-
What stereoisomer of A also gives compound C on heating?
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...

Study smarter with the SolutionInn App


