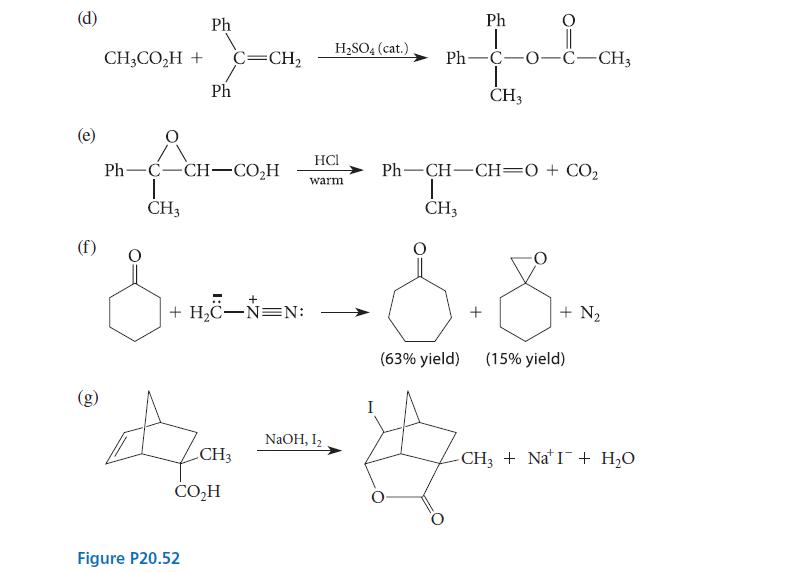
Give a curved-arrow mechanism for each of the reactions given in Fig. P20.52. (a) (b) OEt 1
Question:
Give a curved-arrow mechanism for each of the reactions given in Fig. P20.52.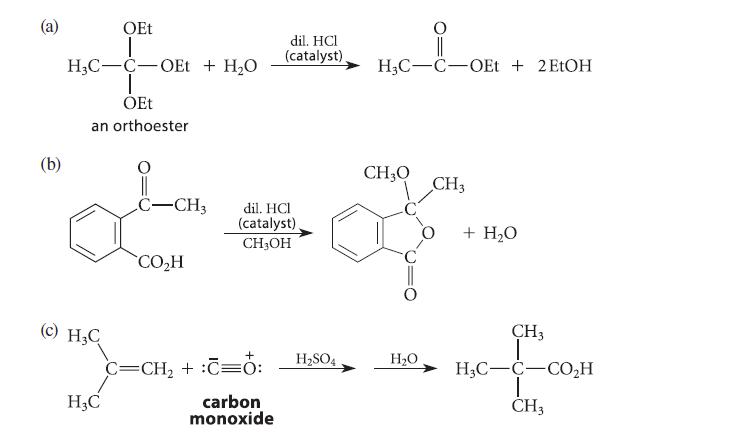
Transcribed Image Text:
(a) (b) OEt 1 H3C-C-OEt + H₂O T OEt an orthoester (c) H₂C H3C C-CH3 CO₂H dil. HCl (catalyst) CH3OH + C=CH₂ + :C=O: dil. HCI (catalyst) carbon monoxide H₂SO4 H₂C-C-OEt + 2 EtOH CH₂O H₂O CH3 + H₂O CH3 HẠC—C—CO,H I CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a b Orthoesters have the same relationship to esters that acetals have to ketones and the hydrolysis mechanisms of both orthoesters and acetals are vi...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give a curved-arrow mechanism for each of the reactions given in Fig. P20.51. OC,H, dil. HCI (catal ,- OC2Hs an orthoester 0 CIH O C CH dil HOI (catalyst) CH OH (e) H,C ,--, carbon monoxide CH Ph Ph...
-
The total amount paid in by__________for the shares they purchase is described as common stock. Select answer from the options below creditors bondholders employees stockholders
-
Draw the simplified curved arrow mechanism for the mechanistic step of (E) -3,5-dimethylhept-3-em-2-one and (CH 3 CH 2 ) 2 CuLi to give the major charged species which is formed. Draw all electrons...
-
Implement and maintain internal control procedures This task will require you to roleplay a meeting with your supervisor, Chris Kohler. You will discuss the reporting requirements and timetables...
-
Refer to the Carolina Communications data in Short Exercises 5-10 and 5-11. Requirement 1. Calculate the gross profit percentage, rate of inventory turnover, and days in inventory ratios for 2012....
-
Noonan and Associates has found from past experience that 25 percent of its services are for cash. The remaining 75 percent are on credit. An aging schedule for accounts receivable reveals the...
-
Assume the same facts as in Activity 10.4 . However, we are also told that the business maintains buffer inventories of 300 units. At what level should the business reorder?6. Hora plc holds...
-
Sardel Company reported net income of $29,975 for 2010. During all of 2010 the company had 1,000 shares of 10%, $100 par, nonconvertible preferred stock outstanding, on which the years dividends had...
-
The following three independent sets of facts relate to contingent liabilities 1. In November of the current year an automobile manufacturing company recalled all pickup trucks manufactured during...
-
Pyridoxal phosphate (PLP) is a form of vitamin B 6 . PLP serves as a coenzyme in the enzyme-catalyzed decarboxylation of meso-2,6-diaminopimelic acid, which is thelast step in the biosynthesis of the...
-
(a) Organolithium reagents such as methyllithium (CH 3 Li) react with carboxylic acids to give ketones. Two equivalents of the lithium reagent are required, and the ketone does not react further....
-
What is the running time of parenthesize(T, T.root( )), as given in Code Fragment 8.26, for a tree T with n nodes? Fragment 8.26 1 /** Prints parenthesized representation of subtree of T rooted at p....
-
Brice Looney owns a small retail ice cream parlor. He is considering expanding the business and has identified two attractive alternatives. One involves purchasing a machine that would enable Mr....
-
A positively charged particle initially at rest on the ground moves \(4.0 \mathrm{~m}\) upward in \(2.00 \mathrm{~s}\). If the particle has a chargeto-mass ratio of \(10 \mu \mathrm{C} / \mathrm{g}\)...
-
Central States Telecom provides communication services in Iowa, Nebraska, the Dakotas, and Montana. Central States purchased goodwill as part of the acquisition of Sheldon Wireless Company, which had...
-
Shown below is selected information from the financial records of Merris Corporation as of December 31: Required a. Determine which of the above items will appear on the statement of cash flows and...
-
Pippa runs a photographic studio specializing in black and white portrait photography. Clients book a one hour studio session and are entitled to receive two large photographs of their choice from...
-
Prove that the n vectors constructed in the proof of Theorem 8.50 are linearly independent and hence form a Jordan basis.
-
During 2012, Cheng Book Store paid $483,000 for land and built a store in Georgetown. Prior to construction, the city of Georgetown charged Cheng $1,300 for a building permit, which Cheng paid. Cheng...
-
Two other hard insecticides (see Problem 13.46) are chlordan and heptachlor. Show how they could be synthesized from cyclopentadiene and hexachlorocyclopentadiene. Cl CI Cl CI CI C Cr C Chlordan CI...
-
Isodrin, an isomer of aldrin, is obtained when cyclopentadiene reacts with the hexachloronorbornadiene, shown here. Propose a structure for isodrin. Cl Cl Cl CI sodrin Cl Cl
-
The following enol (an alkene-alcohol) and keto (a ketone) forms of C2H4O differ in the positions for their electrons, but they are not resonance structures. Explain why they are not. :O C2H4O Enol...
-
Los datos de la columna C tienen caracteres no imprimibles antes y despus de los datos contenidos en cada celda. En la celda G2, ingrese una frmula para eliminar cualquier carcter no imprimible de la...
-
Explain impacts of changing FIFO method to weighted average method in inventory cost valuations? Explain impacts of changing Weighted average method to FIFO method in inventory cost valuations?...
-
A perpetuity makes payments starting five years from today. The first payment is 1000 and each payment thereafter increases by k (in %) (which is less than the effective annual interest rate) per...

Study smarter with the SolutionInn App


