Nitration of phenyl acetate (compound A) results in para substitution of the nitro group. However, nitration of
Question:
Nitration of phenyl acetate (compound A) results in para substitution of the nitro group. However, nitration of dimethyl phenyl phosphate (compound B) results in meta substitution of the nitro group. Suggest a reason that the two compounds nitrate in different positions.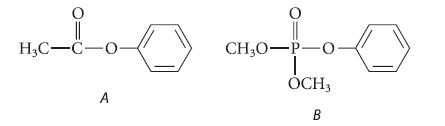
Transcribed Image Text:
H3C-C-O- A 0 CH3O-P-0 OCH3 B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
First draw the resonance structure of compound B that has an electronic octet around phosphorus This ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In an aqueous solution containing sodium bicarbonate, aniline reacts quickly with bromine to give 2, 4, 6-tribromoaniline. Nitration of aniline requires very strong conditions, however, and the...
-
Compounds A and B are carboxylic acids. Identify each one on the basis of its 1H NMR spectrum. (a) Compound A (C3H5ClO2) (Figure 19.10). (b) Compound B (C9H9NO4) has a nitro group attached to an...
-
Compounds A and B are carboxylic acids. Identify each one on the basis of its 1H NMR spectrum. (a) Compound A (C3H5ClO2) (Figure 19.10). (b) Compound B (C9H9NO4) has a nitro group attached to an...
-
Write a StockAccount client that builds an array of StockAccount objects, computes the total value of each account, and prints a report for the accounts with the largest and smallest values. Assume...
-
The combination of physical constants = e2k/hc, where k is the Coulomb constant, is known as the fine-structure constant. It appears in numerous relations in atomic physics. (a) Show that is...
-
Haddleburg Property Management (HPM) offers a variety of services to homeowners. The services include: Outside Care, which includes grass-cutting, snow shovelling, tending of gardens, and so forth;...
-
Explain the purposes of each step of the new-product process.
-
Suppose the central bank of a small country is faced by a rise in the world interest rate, R* what is the effect on its foreign reserve holdings? On its money supply? Can it offset either of these...
-
Medical Tape makes two products: Generic and Label. It estimates it will produce 359,259 units of Generic and 603,571 of Label, and the overhead for each of its cost pools is as follows: Cost Pool...
-
Suppose that the following curved-arrow notation and resonance structures for TPP ylid have been proposed in an attempt to demonstrate that the charges in the ylid can be neutralized by resonance....
-
According to the introduction to this chapter, trade models built exclusively on the idea of comparative advantage have a mixed record when it comes to predicting a countrys trade pattern. Using the...
-
Consider a Yukawa theory with the Lagrangian density \[\begin{equation*}\mathcal{L}=\frac{1}{2} \partial_{\mu} \phi \partial^{\mu} \phi-\frac{1}{2} m^{2} \phi^{2}+\bar{\psi}(i ot \partial-M) \psi-g...
-
Ted sold his Microsoft stock for $40,000 paying a commission of $800. He purchased the stock in 2004 for $8,000 and paid commission of $200. What is the recognized gain on the sale?
-
Liquid water at 80C and at 1atm flows through a heated pipe at a flow rate of 3.1 kg/s. It then leaves the pipe as steam. The water receives 9753840 J of heating from the pipe. Calculate the...
-
The balance sheet of River Electronics Corporation as of December 31, 2023, included 14.00% bonds having a face amount of $90.7 million. The bonds had been issued in 2016 and had a remaining discount...
-
The term mutually exclusive means that two events have no common elements in them. The occurrence of one event means that the other other event does not occur. An example of a mutually exclusive...
-
9a A conical pendulum is made by hanging a mass of 5.0 kg from a large spring of length 1.0 m and spring constant k = 100 N/m. The spring moves in a circle at an angle of 25 deg. When at rest hanging...
-
A fluorescent solid absorbs a photon of ultraviolet light of wavelength 320 nm. If the solid dissipates 0.500 eV of the energy and emits the rest in a single photon, what is the wavelength of the...
-
Prove the formula for (d/dx)(cos-1x) by the same method as for (d/dx)(sin-1x).
-
In Problem 5-3, you drew the enantiomers for a number of chiral compounds. Now go back and designate each asymmetric carbon atom as either (R) or (S). In problem 5.3 (a) (b) (c) (d)...
-
A solution of 2.0 g of (+)-glyceraldehyde, HOCH2-CHOH-CHO, in 10.0 mL of water was placed in a 100-mm cell. Using the sodium D line, a rotation of +1.74o was found at 25 C. Determine the specific...
-
A solution of 0.50 g of (-) - epinephrine (see Figure 5-15) dissolved in 10.0 mL of dilute aqueous HCl was placed in a 20-cm polarimeter tube. Using the sodium D line, the rotation was found to be...
-
Randy (48) takes a $22,000 distribution from his IRA to pay some of his daughter's $28,000 qualified education expenses at an eligible educational institution. His daughter paid $18,000 of her...
-
The takeover specialist chose to use the value derived from dividend discount model, while the directors prefer to use Net Realisable Value approach. Critically discuss the reasoning of each parties...
-
Tony and Suzie graduate from college in May 2021 and begin developing their new business. They begin by offering clinics for basic outdoor activities such as mountain biking or kayaking. Upon...

Study smarter with the SolutionInn App


