Which of the following are Fischer projections of a meso compound? - - - CH-0 - -OH
Question:
Which of the following are Fischer projections of a meso compound? 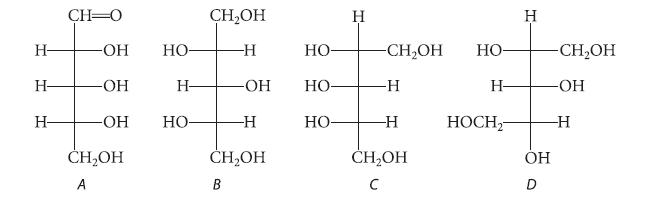
Transcribed Image Text:
Н- Н- Н- CH-0 -ОН -OH -ОН CH₂OH A НО Н- но- CH₂OH -Н -OH -Н CH₂OH B но- но- НО- Н -CH₂OH -Н -Н CH₂OH с НО- Н- HOCH₂ Н ОН -CH₂OH -ОН -Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
It is relatively simple to determine if a compound is meso using Fisher projections First perform a ...View the full answer

Answered By

Ujjwal Ghosh
I have been teaching for last 10 years. Many students whom I've taught are now studying the courses of their choice. Most of my students cracked the competitive examinations in the first attempt.
I've taught many students from graduation to higher level like master degree courses.
They were very happy with my teaching style.
I can solve higher order thinking questions in very less time.
https://www.linkedin.com/in/ujjwal-ghosh-1a506a1b4/
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Following are Fischer projections for a group of five-carbon sugars, all of which are aldopentoses. Identify the pairs that are enantiomers and the pairs that are epimers. (The sugars shown here are...
-
Which of the following compounds are chiral? Draw each compound in its most symmetric conformation, star (*) any asymmetric carbon atoms, and draw any mirror planes. Label any meso compounds. You may...
-
Which compound is not a meso compound? (a) (b) (c) (d) (e) Cl Cl CH3 Cl Cl Cl
-
An array is bitonic if it consists of an increasing sequence of keys followed immediately by a decreasing sequence of keys. Given a bitonic array, design a logarithmic algorithm to find the index of...
-
The K 0 particle has a rest mass of 497.7 MeV/c 2 . It decays into a and + , each with rest mass 139.6 MeV/c 2 . Following the decay of a K 0 , one of the pions is at rest in the laboratory....
-
A 2.4-m-diameter tank is initially filled with water 4 m above the center of a sharp-edged 10-cm-diameter orifice. The tank water surface is open to the atmosphere, and the orifice drains to the...
-
12-5. What is the principal distinction between a corporate vertical marketing system and an administered vertical marketing system?
-
Macro Media, LLC, has three members: WLKT Partners, Amanda Nelson, and Daily Sentinel Newspaper, LLC. On January 1, 2012, the three members had equity of $250,000, $50,000, and $140,000,...
-
BACK NEX Multiple Choice Question 49 In 2019, Sheffield Corp. sold 6600 units at $600 each. Variable expenses were $420 per unit, and fed expenses were $360000. The same selling price, variable...
-
Which pair of the following aldoses are epimers and which pair are enantiomers? H HO H CH=0 -OH A - -OH -CHOH 0=CH- - OH H B - -H -OH -CHOH H - OH -CH=0 -OH - - CHOH
-
Name each of the following aldoses. In part (a), work back to the Fischer projection and consult Fig. 24.3. In part (b), decide which carbons have configurations different from those of glucose, and...
-
Use Newton?s law to compute the gravitational acceleration g(r) as a function of radius r within a sphere of total mass M that is uniformly distributed through the volume. Compute g(r) in a simple...
-
Prove that Eq. (19.34) gives the simplest multi-gluon and gluon-quark states that contain an \(\mathrm{SU}(3)\) color singlet in the decomposition. Data from Eq. 19.34 (GG)1: (88)1 (Gqq) : [8 (383)8]...
-
In question 70, what is the probability that of the 100 cars test-driven, more than 35 cars get more than 45 miles per gallon? How many of the 100 cars tested would you expect to get more than 45...
-
Construct the braid group products (a) (b) using the algorithm of Fig. 29.16 . Data from Fig. 29.16
-
Worksheet The adjusted trial balance columns of a worksheet for Bond Corporation are shown below. The worksheet is prepared for the year ended December 31. Complete the worksheet by (a) entering the...
-
The Healthy Catering Service had the following transactions in July, its first month of operations: 1 Kelly Foster contributed \(\$ 18,000\) of personal funds to the business in exchange for common...
-
In an optically pumped laser, the light that causes optical pumping is always shorter in wavelength than the laser beam. Explain.
-
Discuss the information available from the following techniques in the analysis of inorganic pigments used in antique oil paintings: (i) Powder X-ray diffraction, (ii) Infrared and Raman...
-
Circle any lone pairs (pairs of nonbonding electrons) in the structures you drew for Problem 1-3. In problem (a) N2 (b) HCN (c) HONO (d) CO2 (e) CH3CHNH (f) HCO2H (g) C2H3CI (h) HNNH
-
In the following sets of resonance forms, label the major and minor contributors and state which structures would be of equal energy. Add any missing resonance forms. (a) (b) (c) (d) (e) CH,_...
-
For each pair of ions, determine which ion is more stable. Use resonance forms to explain your answers. (a) (b) (c) (d) (e) CH CHCH or CH CH OCH CH CH CH-CH or CH CH CH2 CH CH,_ CH, or CH,-C N: CH2...
-
Nitin is paid a base salary of $200 per week and commission at the rate of 3% for sales over $5000, 4% if his sales are over $8000, and 5% if sales are over $15,000. How much will Nitin earn in a...
-
Safa is paid a base salary of $1500 per month and a commission of 6% on all sales over $75,000. Last month, Safa's gross salary was $4440. What were her sales for the month? a$149,000 b$124,000...
-
Your regular hourly rate of pay is $15.86, and you are paid double time for all work on weekends and for any time over forty hours per week (Monday to Friday). Calculate your gross earnings for a...

Study smarter with the SolutionInn App


