Consider the following mechanism for Eq. 18.19. Identify the process associated with each step. Counting electrons at
Question:
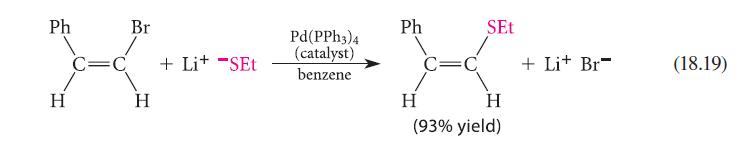
Consider the following mechanism for Eq. 18.19. Identify the process associated with each step. Counting electrons at each stage may help you.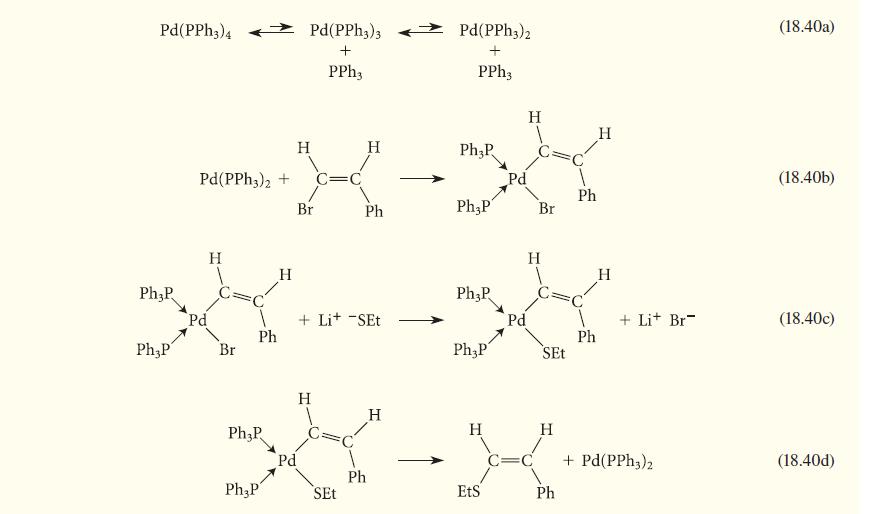
Transcribed Image Text:
Pd(PPH3)4 Ph3P Ph,P Pd(PPh 3)2 + Pd H Br Ph H Ph₂P Pd(PPH3)3 + PPh3 H Br C=C H Ph + Lit -SET Pd(PPH3)2 + PPh3 Ph₂P Ph,P Ph₂P Ph P Pd Pd H Br H SEt XXX-X Ph Ph H H + Lit Br + Pd(PPH3)2 (18.40a) (18.40b) (18.40c) (18.40d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Step 1840a consists of two successive ligand dissociations that reduce the ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the process evaluation article that you chose and explain why you selected this example. Describe the purpose of the evaluation, the informants, the questions asked, and the results of the...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Dickens, Kristen, is enrolled as a doctoral student in the Counselor Education at the University of New Orleans. She is a registered counselor intern in the state of Louisiana and works at a...
-
Implement the method contains() for HashST.
-
1. For the principal quantum number n = 3, what are the possible values of the quantum numbers and m? 2. What is the energy of the shortest wavelength photon emitted by the hydrogen atom?
-
Compute the determinants using cofactor expansion along any row or column that seems convenient. sin 0 sin 0 cos 0 tan 0 cos 0 - cos 0 sin 0
-
Develop a hypothetical ethical dilemma for a conflict-of-interest situation. AppendixLO1
-
Danas Ribbon World makes award rosettes. Following is information about the company: Variable cost per rosette ...... $ 1.60 Sales price per rosette ...... 3.00 Total fixed costs per month .......
-
Please answer asap will upvote Colt Company owns a machine that can produce two specialized products Production time for Product TLX is three units per hour and for Product MTV is five units per...
-
Arrange the following compounds according to increasing rate of elimination with NaOEt in EtOH. What is the product in each case? Ph H C=C A Ph Br Ph H C=C B Br Ph Ph H C=C C Ph Cl H H =C D Br Ph
-
The reaction given in Fig. P17.59 occurs by a mechanism called the S N 2 mechanism, which is a bimolecular substitution that occurs by reaction of the nucleophile at an allylic carbon. In this...
-
Find the centroid of the region lying underneath the graph of the function over the give interval. (x) = x, [1, 4]
-
Gilbert Canned Produce (GCP) packs and sells three varieties of canned produce: green beans; sweet peas; and tomatoes. The company is currently operating at 82 percent of capacity. Worried about the...
-
Apply at least two of the theories (of your choice) to your personal experience? The theories are Leader-Member Exchange Theory (LMX Model), the Situational Leadership Model, the Contingency Model...
-
Game theory is used in economics, social science and computer science to understand and predict the behaviour of people and intelligent entities. In project management and business scenarios, it can...
-
During a chemistry lab, you take a 0.2 kg sample of ice and put it in a beaker with a thermometer. You then place the beaker with the ice on a hot plate, and turn on the hot plate. This hot plate...
-
Selected information from Carla Vista Ltd.'s statement of financial position and statement of income is as follows: Carla Vista Ltd. Statement of Financial Position (partial) December 31 2024 2023...
-
An electron-positron pair is created in a particle detector. If the tracks of the particles indicate that each one has a kinetic energy of 0.22 MeV, what is the energy of the photon that created the...
-
Let X be a random variable taking on values a1, a2, . . . , pr with probabilities p1, p2, . . . , pr and with E(X) = μ. Define the spread of X as follows: This, like the standard deviation, is a...
-
A chemist finds that the addition of (+)-epinephrine to the catalytic reduction of butan-2-one (Figure 5-16) gives a product that is slightly optically active, with a specific rotation of +0.45o...
-
1. Make a model of each compound, draw it in its most symmetric conformation, and determine whether it is capable of showing optical activity. (a) 1-bromo-1-chloroethane (b) 1-bromo-2-chloroethane...
-
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through...
-
Nitin is paid a base salary of $200 per week and commission at the rate of 3% for sales over $5000, 4% if his sales are over $8000, and 5% if sales are over $15,000. How much will Nitin earn in a...
-
Safa is paid a base salary of $1500 per month and a commission of 6% on all sales over $75,000. Last month, Safa's gross salary was $4440. What were her sales for the month? a$149,000 b$124,000...
-
Your regular hourly rate of pay is $15.86, and you are paid double time for all work on weekends and for any time over forty hours per week (Monday to Friday). Calculate your gross earnings for a...

Study smarter with the SolutionInn App


