From the specific rotations shown in Eq. 24.18, calculate the percentages of - and -D- glucopyranose present
Question:
From the specific rotations shown in Eq. 24.18, calculate the percentages of α- and β-D- glucopyranose present at equilibrium.Compare your answer to the data given in Table 24.1.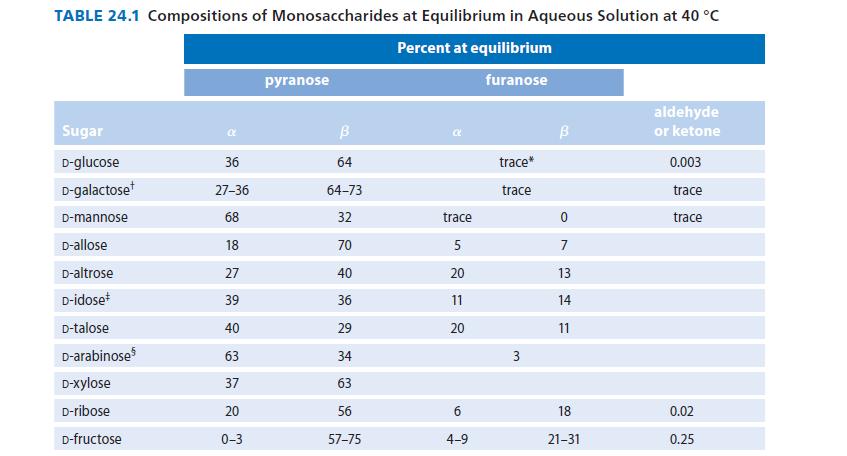
![HO HOCH HO OH -H OH a-anomer [a] =+112 degrees ml g-1 dm- acid or base H20 HOCH HO OH equilibrium mixture:](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/9/3/2/84265716f2a8015e1701932840806.jpg)
Transcribed Image Text:
TABLE 24.1 Compositions of Monosaccharides at Equilibrium in Aqueous Solution at 40 °C Percent at equilibrium furanose Sugar D-glucose D-galactose D-mannose D-allose D-altrose D-idose D-talose D-arabinose D-Xylose D-ribose D-fructose 36 27-36 68 18 27 39 40 63 37 20 0-3 pyranose B 64 64-73 32 70 40 36 29 34 63 56 57-75 trace 5 20 11 20 6 4-9 trace* trace 3 В 0 7 13 14 11 18 21-31 aldehyde or ketone 0.003 trace trace 0.02 0.25
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Each form of the sugar contributes its own optical rotation in proportion to the amoun...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What are the real and anticipated arguments that could be made by those at Harrison Industries who may try to convince Donna to go along with the accounting for future severance payments? Include...
-
Calculate the percentages of -D-glucose and -D-glucose present at equilibrium from the specific rotations of -D-glucose, -D-glucose, and the equilibrium mixture. Compare your values with those given...
-
The two most common isotopes of uranium are 235U and 238U. (a) Compare the number of protons, the number of electrons, and the number of neutrons in atoms of these two isotopes. (b) Using the...
-
Hansel Electronics has the following: If Hansel has 7,000 units on hand at December 31, the cost of ending inventory under the average-cost method is: (a) $84,000. (b) $70,000. (c) $56,000. (d)...
-
The Lorentz transformation for y and z is the same as the classical result: y = y and z = z. Yet the relativistic velocity transformation does not give the classical result uy = uy and uz = uz....
-
Water at 15°C (r = 999.1 kg/m 3 and μ = 1.138 à 10 -3 kg/m·s) is flowing steadily in a 30-m-long and 5-cm-diameter horizontal pipe made of stainless steel at a rate of...
-
Describe factors that marketing executives consider when selecting and managing a marketing channel.
-
Consider a long bar of square cross section (0.8 m to the side) and of thermal conductivity 2 W/m K. Three of these sides are maintained at a uniform temperature of 300C. The fourth side is exposed...
-
Required information Problem 10-8 (Algo) Nonmonetary exchange (LO10-6) (The following information applies to the questions displayed below.) Case A. Kapono Farms exchanged an old tractor for a newer...
-
Using the curved-arrow notation, fill in the details for base-catalyzed mutarotation of glucopyranose. Begin by removing a proton from the hydroxy group at carbon-1.
-
Using the curved-arrow notation, fill in the details for acid-catalyzed mutarotation of glucopyranose shown in Eq. 24.19. Begin by protonating the ring oxygen. HO HO HOCH2 a-anomer OH HO opening of...
-
What is the difference between a Chapter 7 bankruptcy and a Chapter 11 bankruptcy?
-
Suppose a small flashlight bulb is on the bottom of the bathtub of Problem 19, directly under the toy boat. When this bulb is lit and the ceiling light is turned off, how does the size of the shadow...
-
Draw a scatter diagram and find \(r\) for the data shown in each table in Problems 25-30. X 85 90 y 80 40 100 30 102 28 105 25
-
Rothera Point Utilities (RPU) provides customers with 7 million megawatt-hours (MWh) of electricity each year. RPU operates three different generation facilities to meet this demand: the Rothera...
-
Explain the components of the path evaluation function f(node) used by A*. Do you think it is the best evaluation function that could be used? To what kinds of problems might it be best suited? And...
-
Celvin FoodStuff operates a chain of mini conve- nience stores in downtown city settings, offering beverages, snack food, and some fresh food items to passing pedestrian traffic. A typical Celvin...
-
A scanning electron microscope is used to look at cell structure with 10-nm resolution. A beam of electrons from a hot filament is accelerated with a voltage of 12 kV and then focused to a small spot...
-
Tell whether the angles or sides are corresponding angles, corresponding sides, or neither. AC and JK
-
For each compound, state whether its bonding is covalent, ionic, or a mixture of covalent and ionic. (a) NaCl (b) NaOH (c) CH3Li (d) CH2CI2 (e) NaOCH3 (f) HCO2Na (g) CF4
-
(a) Both PCI3 and PCI5 are stable compounds. Draw Lewis structures for these two compounds. (b) NCI3 is a known compound, but all attempts to synthesize NCI5 have failed. Draw Lewis structures for...
-
Draw a Lewis structure for each species. (a) N2H4 (b) N2H2 (c) (CH3)2NH2CI (d) CH3CN (e) CH3CHO (f) CH3S(O)CH3 (g) H2SO4 (h) CH3NCO (i) CH3OSO2OCH3 (j) CH3C(NH)CH3 (k) (CH3)3CNO
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


