Using the curved-arrow notation, fill in the details for acid-catalyzed mutarotation of glucopyranose shown in Eq. 24.19.
Question:
Using the curved-arrow notation, fill in the details for acid-catalyzed mutarotation of glucopyranose shown in Eq. 24.19. Begin by protonating the ring oxygen.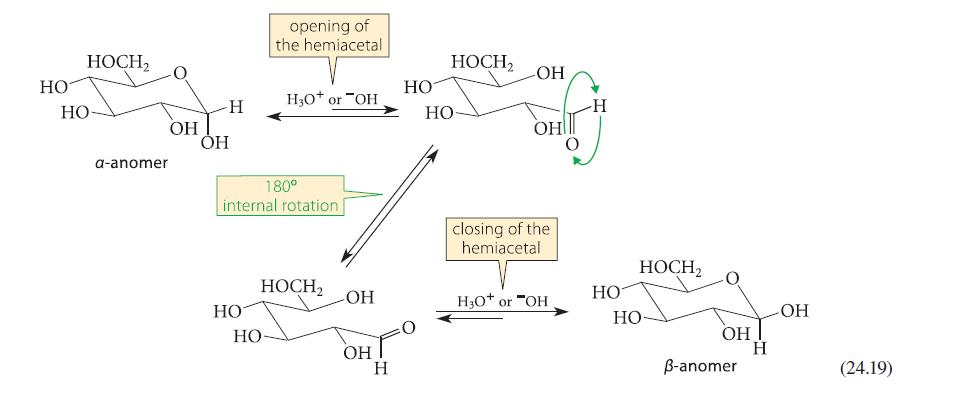
Transcribed Image Text:
HO HO HOCH2 a-anomer ОН OH Н HO opening of the hemiacetal 180° internal rotation HO H3O+ or OH HOCH₂ НО OH он о Н HOCH₂ НО -ОН OH closing of the hemiacetal H3O+ or "OH НО HOCH₂ НО ОН B-anomer H -OH (24.19)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The following mechanism begins with the aanomer proton...View the full answer

Answered By

HABIBULLAH HABIBULLAH
I have been tutor on chegg for approx 5 months and had solved a lot of questions.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Read the New York Times article "Bangladesh fears an exodus of apparel firms" and answer the question What if any is your opinion, as a consumer, toward these types of horrific events? Do you change...
-
Draw the curved arrow notation and the product for the nucleophilic addition of a propyl anion to cyclohexanone. Draw the curved arrow notation Draw the product with lone pairs and nonzero formal...
-
Poppins Company has the following: If 9,000 units are on hand at December 31, the cost of the ending inventory under FIFO is: (a) $99,000. (b) $108,000. (c) $113,000. (d) $117,000. Inventory, Jan. 1...
-
A horizontal turntable rotates with angular speed . There is a clock at the center of the turntable and one at a distance r from the center. In an inertial reference frame, the clock at distance r is...
-
Consider laminar flow of a fluid through a square channel with smooth surfaces. Now the average velocity of the fluid is doubled. Determine the change in the head loss of the fluid. Assume the flow...
-
Explain what supply chain and logistics management are and how they relate to marketing strategy.
-
The following information, in T-account format, is provided for Mars Company for the year 2012: Additional information: a. Sales revenue for the period was $164,000. Accounts receivable (net)...
-
Newark Incorporated, which follows ASPE, has a March 31 year-end. The 2021 fiscal year was particularly lucrative for Newark; they realized sales of $660,000 with cost of goods sold of only $363,000...
-
From the specific rotations shown in Eq. 24.18, calculate the percentages of - and -D- glucopyranose present at equilibrium.Compare your answer to the data given in Table 24.1. TABLE 24.1...
-
Draw a Fischer projection, a Haworth projection, and a line-and wedge structure for each of the following compounds. For the pyranoses, draw the two possible chain conformations. (a) - D...
-
The Harvey Corporation is considering a change in its cash-only policy. The new terms would be net one period. Based on the following information, determine if Harvey should proceed or not. The...
-
Convex Productions produces full-length motion pictures for distribution worldwide. Convex has just purchased the rights to a movie script entitled Native Sun, which it intends to develop as its next...
-
You are visiting the Engineering Office of Denton Hospital, as part of a consulting project. You notice some charts on one wall which look familiar to you: One of the employees notices you reading...
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
To resolve details of an object, you must use a wavelength that is about the same size, or smaller, than the details you want to observe. Suppose you want to study a molecule that is about 1.000 ...
-
Drainee purchases direct materials each month. Its payment history shows that 65% is paid in the month of purchase with the remaining balance paid the month after purchase. Prepare a cash payment...
-
Draw a Lewis structure for each compound. Include all nonbonding pairs of electrons. (a) CH3COCH2CHCHCOOH (b) NCCH2COCH2CHO (c) CH2CHCH(OH)CH2CO2H (d) CH2CHC(CH3)CHCOOCH3
-
Draw a line-angle formula for each compound in Problem 1-26. In problem (a) CH3COCH2CHCHCOOH (b) NCCH2COCH2CHO (c) CH2CHCH(OH)CH2CO2H (d) CH2CHC(CH3)CHCOOCH3
-
Draw Lewis structures for (a) Two compounds of formula C4H10 (b) Two compounds of formula C2H6O (c) Two compounds of formula C2H7N (d) Three compounds of formula C2H7NO (e) Three compounds of formula...
-
September 23 for $1,050 each. On December 24 , it sold one of the diamonds that was purchased on July 9 . Using the specific identification method, its ending inventory (after the December 24 sale)...
-
Madsen Motors's bonds have 13 years remaining to maturity. Interest is paid annually, they have a $1,000 par value, the coupon interest rate is 8%, and the yield to maturity is 10%. What is the...
-
Builder Products, Incorporated, uses the weighted - average method in its process costing system. It manufactures a caulking compound that goes through three processing stages prior to completion....

Study smarter with the SolutionInn App


