Determine whether the following carbohydrate derivative, shown in Fischer projection, has the D or L configuration. HOCH-
Question:
Determine whether the following carbohydrate derivative, shown in Fischer projection, has the D or L configuration.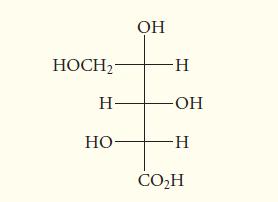
Transcribed Image Text:
HOCH₂- Н- но- OH -Н -ОН -Н CO₂H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
First redraw the structure so that the carbon with the lowest number ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine whether each of the following carbohydrates is a d sugar or an l sugar, and assign a configuration for each chirality center. After assigning the configuration for all of the...
-
Determine whether the following integrals are convergent or divergent. (Define the integrands to be 0 where they are not already defined.) (a) (b) (c) (d) (e) (f) sinx dx x32 cos x dx l I In xdx In...
-
Determine whether each statements is true or false: (a) A racemic mixture of enantiomers is optically inactive. (b) A meso compound will have exactly one non-super-imposable mirror image. (c)...
-
Develop a data type ResizingArrayQueueOfStrings that implements a queue with a fixed-length array in such a way that all operations take constant time. Then, extend your implementation to use a...
-
A particle with momentum of 6 MeV/c has total energy of 8 MeV. (a) Determine the rest mass of the particle. (b) What is the energy of the particle in a reference frame in which its momentum is 4...
-
The U.S. Fish and Wildlife Service has considered banning imports of beluga caviar to protect the beluga sturgeon in the Caspian and Black seas, whose sturgeon populations have fallen 90% in the last...
-
2 Suppose the president of a carpet manufacturing firm has asked you to look into the possibility of bypassing the firms wholesalers (who sell to carpet, department, and furniture stores) and selling...
-
The questions in this exercise are based on the Benetton Group, a company headquartered in Italy and known in the United States primarily for one of its brands of fashion apparelUnited Colors of...
-
The budgeted unit sales of Weller Company for the upcoming fiscal year are provided below: 1st Quarter 2nd Quarter 3rd Quarter 4th Quarter Budgeted unit sales 15,000 16,000 14,000 13,000 The companys...
-
When 1,5-dibromopentane reacts with ammonia, among several products isolated is a water- soluble compound A that rapidly gives a precipitate of AgBr with acidic AgNO 3 solution. Compound A is...
-
A compound has IR absorptions at 34003500 cm 1 and the following NMR spectrum: 2.07 (6H, s), 2.16 (3H, s), 3.19 (broad, exchanges with D 2 O), 6.63 (2H, s). To which one of the following...
-
The following is a set of data from a sample of n = 5: 7 - 5 - 8 7 9 a. Compute the first quartile (Q1), the third quartile (Q3), and the interquartile range. b. List the five- number summary. c....
-
Recall from Case 1.2 that Auto Concepts is a new division of a large automobile manufacturer that has been slowly losing market share to its competitors. Auto Concepts was created to reclaim the...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a diverging lens. \((b)\) Is the image real or virtual? (c) Is it upright or...
-
Show that the ray exiting the block in Figure P33.53 is parallel to the ray entering the block. Data from Figure P33.53
-
The element is subjected to the state of stress shown. If the material is machine steel having a yield stress of \(\sigma_{Y}=750 \mathrm{MPa}\), determine the factor of safety with respect to...
-
Determine the vertical displacement of the ring at point \(B\). \(E I\) is constant. B P A
-
When aluminum is exposed to oxygen, a very thin layer of aluminum oxide forms on the outside. Aluminum oxide is a good insulator. Nevertheless, if two aluminum wires are twisted together, electric...
-
a. Show that the expansion of q(x) in ascending powers of x can be approximated to 10 2x + Bx 2 + Cx 3 where B and C are constants to be found. b. Find the percentage error made in using the series...
-
Draw complete Lewis structures for the following condensed structural formulas. (a) CH3(CH2)3CH(CH3)2 (b) (CH3)2CHCH2CI (c) CH3CH2CH(CH3)2 (d) CH2CHCHO (e) (CH3)3CCOCHCH2 (f) CH3COCOOH (g) 1CH3CH2...
-
(a) Use your molecular models to make ethane, and compare the model with the preceding structures. (b) Make a model of propane (C3H8), and draw this model using dashed lines and wedges to represent...
-
Two isomers of 1,2-dichloroethene are known. One has a dipole moment of 2.4 D; the other has zero dipole moment. Draw the two isomers and explain why one has zero dipole moment. CHCl=CHCl 1,...
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


