Consider the detailed structure of the semiquinone? QH shown in Eq. 18.79a. (a) There are two possible
Question:
Consider the detailed structure of the semiquinone? QH shown in Eq. 18.79a.
(a) There are two possible structures for this semiquinone; draw them both.
(b) Show the resonance structures for either of the structures you gave in part (a).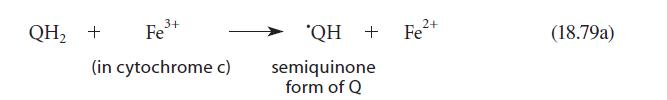
Transcribed Image Text:
QH₂ + 3+ Fe³+ (in cytochrome c) 'QH + Fe²+ semiquinone form of Q (18.79a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a CH3O CH30 0 CH3 H 10 or CH3O ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
There are two possible structures of XeF2Cl2, where Xe is the central atom. Draw them, and describe how measurements of dipole moments might be used to distinguish among them.
-
Consider the following reactions. For parts b-d, reference Exercise 58. a. When C5H12 is reacted with Cl2(g) in the presence of ultraviolet light, four different monochlorination products form. What...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Show that an emission tax and an absolute emission standard are equivalent instruments to regulate a polluting monopolist if and only if the standard is binding.
-
How fast must you be moving toward a red light ( = 650 nm) for it to appear green ( = 525 nm)?
-
From the interest tables in Appendix B, determine the value of the following factors by interpolation, and compare the results with those obtained from evaluating the A/P and P/A interest formulas:...
-
What is the relationship between leadership and management? AppendixLO1
-
Gary Stewart and his wife Debbie, both age 32, have been married for twelve years and have a 2- year old son, Grant. Gary is a mid-level manager at a Fortune 500 company and currently earns $85,000...
-
In January 2022, the management of Handley Corporation, a publicly-traded company, decides that it has sufficient cash to purchase some debt and equity securities to be held as trading investments....
-
Draw the important resonance structures of the radicals formed when each of the following reacts with R?, a general free radical. (a) Vitamin E (b) BHT
-
(a) Using the fishhook notation, derive the important resonance structures of the vitamin C-derived radical in Eq. 18.81b. (b) In the laboratory, the radical derived from vitamin E can react with a...
-
One of the chain-termination steps that sometimes occurs to interrupt polymerization is the following reaction between two radicals. Propose a mechanism for the reaction, using fishhook arrows to...
-
Sketch plane / intersecting plane K. Then draw a line & in plane J that intersects plane Kat a single point. A X C B D E
-
Use a graphing utility to verify any five of the graphs that you drew by hand in Exercises 126. Data from exercise 1-26 1. x + 2y = 8 3. x2y> 10 2. 3x6y 12 4. 2xy > 4
-
The following information pertains to Porter Company for 2011. Ending inventory consisted of 30 units. Porter sold 320 units at \(\$ 30\) each. All purchases and sales were made with cash. Required...
-
In Problems 7780, use a numerical integration routine on a graphing calculator to find the area bounded by the graphs of the indicated equations over the given interval (when stated). Compute answers...
-
Solar Heating, Inc., had the following transactions for 2011: Required a. Determine the quantity and dollar amount of inventory at the end of the year, assuming Solar Heating Inc. uses the FIFO cost...
-
Photons of wavelength 350 nm are incident on a metal plate in a photocell and electrons are ejected. A stopping potential of 1.10 V is able to just prevent any of the ejected electrons from reaching...
-
Research corporate acquisitions using Web resources and then answer the following questions: Why do firms purchase other corporations? Do firms pay too much for the acquired corporation? Why do so...
-
Draw the most stable conformation of: (a) ethylcyclohexane (b) 3-isopropyl-1,1-dimethylcyclohexane (c) cis-1-tert-butyl-4-isopropylcyclohexane
-
(a) Draw both chair conformations of cis-1,4-dimethylcyclohexane, and determine which conformer is more stable. (b) Repeat for the trans isomer. (c) Predict which isomer (cis or trans) is more stable.
-
Use your results from Problem 3-25 to complete the following table. Each entry shows the positions of two groups arranged as shown. For example, two groups that are trans on adjacent carbons...
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


