Bearing in mind the hydrolysis reaction discussed in Problem 22.94, predict the final product of the reaction
Question:
Bearing in mind the hydrolysis reaction discussed in Problem 22.94, predict the final product of the reaction sequence shown in Fig. P22.95.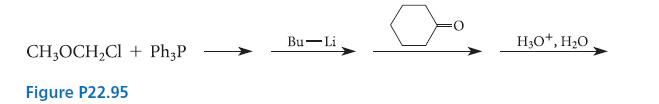
Transcribed Image Text:
CH3OCH₂Cl + Ph3P Figure P22.95 Bu-Li H3O+, H₂O,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
The Wittig reaction gives a vinylic ether which as shown i...View the full answer

Answered By

Zablon Gicharu
I am an educator who possesses the requisite skills and knowledge due to interacting with students for an extended period. I provide solutions to various problems in step-by-step explanations, a well-thought approach and an understandable breakdown. My goal is to impart more straightforward methodologies and understanding to students for more remarkable achievements.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the 1980s, Sears acquired several financial services firms, including Allstate Insurance and Dean Witter Brokerage Services. Sears kept these businesses as largely autonomous divisions. By 1994,...
-
In the 19805. Sears acquired several financial services firms including Allstate Insurance and Dean Witter Brokerage Services. Seas kept these business as largely autommous divisions. By 1995, the...
-
The raw data for Problem 22, from Freund (1979), appear below. For the model of Problem 22, a. Fit the model. b. What discrepancies do you note between the results of (a) and the data summary...
-
Draw the BST that results when you insert the keys E A S Y QUE S T I O N in that order into an initially empty tree. What is the height of the resulting BST?
-
Find the ratio of the total energy to the rest energy of a particle of rest mass m0 moving with speed (a) 0.1c, (b) 0.5c, (c) 0.8c, and (d) 0.99c.
-
Patrick, the owner, makes the same offer to the manager at each of his stores: At the end of the year, pay me a lump-sum of $100,000, and you can keep any additional profit. Astrid, a manager at one...
-
Does your marketing plan involve selecting channels and intermediaries? If the answer is no, read no further and do not include this element in your plan. If the answer is yes: 1 Identify which...
-
Top Notch Company purchased merchandise on account from a supplier for $13,500, terms 2/10, n/30. Top Notch Company returned $4,000 of the merchandise before payment was made and received full...
-
A. Journalize the closing entries. (Please note that pages 1, 2, and 3 show the transactions and adjusting entries that were recorded in Continuing Problems 2 and 3.) Refer to the Chart of Accounts...
-
Outline a synthesis of p-dipropylaminoacetophenone from chlorobenzene.
-
Suggest a sequence of reactions for carrying out each of the following conversions. (a) Benzoic acid to Ph 3 COH (triphenylmethanol) (b) Butyric acid to 3-methyl-3-hexanol (c) Isobutyronitrile to...
-
Write a paper on one Latin American country. Address the following components, focusing on how these elements result in an opportunity for the selected country BRAZIL to thrive in the new global...
-
Sample grade point averages for ten male students and ten female students are listed. Males 2.4 3.7 3.8 3.9 Females 2.8 3.7 2.1 3.9 2.8 2.6 3.6 3.3 4.0 1.9 3.6 4.0 2.0 3.9 3.7 2.3
-
Fill in the columns in the following table. What quantity should a profit-maximizing firm produce? Verify your answer with marginal reasoning. 9 0 1 2 3 st 4 5 6 TFC $5 5 5 5 5 5 5 TVC $0 3 5 9 16 25...
-
Perform the experiments in Problems 48-51, tally your results, and calculate the probabilities (to the nearest hundredth). Flip three coins simultaneously 100 times, and note the results. The...
-
The following information is available for Spring Inc. and Winter Inc. at December 31, 2011: Required a. What is the accounts receivable turnover for each of the companies for 2011? b. What is the...
-
Margin of error = 0.5 g, standard deviation = 8.7 g
-
Why does a confined particle have quantized energy levels?
-
Could the owner of a business prepare a statement of financial position on 9 December or 23 June or today?
-
For each of the following compounds and ions, 1. Draw a Lewis structure. 2. Show the kinds of orbitals that overlap to form each bond. 3. Give approximate bond angles around each atom except...
-
In most amines, the nitrogen atom sp3 is hybridized, with a pyramidal structure and bond angles close to 109°. In urea, both nitrogen atoms are found to be planar, with bond angles close to...
-
Predict the hybridization, geometry, and bond angles for the central atoms in: (a) But-2-ene, CH3CH=CHCH3 (b) CH3CH=NH
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


