Calculate the first-order correction to the energy of an electron in electron volts eV, in the ground
Question:
Calculate the first-order correction to the energy of an electron in electron volts eV, in the ground state of hydrogen due to the gravitational potential of the nucleus given by 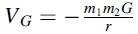 where m1 and m2 are electron and proton masses, respectively, and G is the gravitational constant given by G= 6.672.10-11N.m2.kg-2.
where m1 and m2 are electron and proton masses, respectively, and G is the gravitational constant given by G= 6.672.10-11N.m2.kg-2.
Transcribed Image Text:
VG = - m;m2G
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The firstorder correction to the energy of an electron in the ground state of hydrogen due to the gr...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The kinetic energy of an electron in a particular Bohr orbit of hydrogen is 1.35 10-19 J. (a) Which Bohr orbit does the electron occupy? (b) Suppose the electron moves away from the nucleus to the...
-
The rest energy of an electron is 0.511 MeV. What momentum (in MeV/c) must an electron have in order that its total energy be 3.00 times its rest energy?
-
The de Broglie wavelength of an electron in a hydrogen atom is 1.66 nm. Identify the integer n that corresponds to its orbit.
-
Draw a UML Sequence Diagram for the process involved in paying a vendor for an item in cash. The process to follow is below. [15 points] a) Go to the bank b) Request your account balance c) The bank...
-
Fellowes and Associates Chartered Accountants is a successful mid-tier accounting firm with a large range of clients across Canada. During 2011, Fellowes and Associates gained a new client, Health...
-
A 1.00-kg block of copper at 20.0C is dropped into a large vessel of liquid nitrogen at 77.3 K. How many kilograms of nitrogen boil away by the time the copper reaches 77.3 K? (The specific heat of...
-
3. What are foreign currency commitments? Why are they considered special?
-
The Miller Corporation acquired 30% of the outstanding common stock of the Crowell Corporation for $160,000 on January 1, 2007 and obtained significant influence. The purchase price of the shares was...
-
Compute the monthly payment of a $300,000,30-year mortgage at 4.0% APR. 1,446 1,606 1,685 1,755 1,432
-
Forestry Inc. (FI) is a private company that operates in the forest products business. It has two divisions. The first division is the forest operations where the company owns and grows large tracts...
-
We have seen that in a magnetic field, the magnetic moment of an electron couples to an external magnetic field B to give the so-called Zeeman term H Z =-gB. Bzsz where for free electrons the factor...
-
Equations of motion of an electron in the presence of an electric field. (a) Calculate the velocity of the electron at k = /a. (b) If the electric field E is applied in the -x direction, derive the...
-
Describe, in words, how to use the variable growth rate technique to value a stock.
-
The equation for the standard normal curve (the normal curve with mean 0 and standard deviation 1) graphs as an exponential curve. Graph this curve, whose equation is \[y=\frac{e^{-x^{2} /...
-
Design an undirected network with N=7 and L=12. Based on how you drew your network, classify it as either fully connected ,random, or scale-free. Justify your decision with a short paragraph response.
-
Use the Ch08_AviaCo database shown in Figure P8.35 to work Problems 3546. Modify the MODEL table to add the attribute and insert the values shown in the following table. Table P8.35 Attribute and...
-
The Tip Calculator app does not need a Button to perform its calculations. Reimplement this app to use property listeners to perform the calculations whenever the user modifies the bill amount or...
-
A particle, carrying a positive charge of \(4 \mathrm{nC}\), located at \((5 \mathrm{~cm}, 0)\) on the \(x\)-axis experiences an attractive force of magnitude 115.2 \(\mathrm{N}\) due to an unknown...
-
Why do U.S. corporations go international?
-
Ann hires a nanny to watch her two children while she works at a local hospital. She pays the 19 year-old nanny $125 per week for 48 weeks during the current year. a. What is the employers portion of...
-
How many milliliters of 0.800 M KOH should be added to 5.02 g of 1,5-pentanedioic acid (C 5 H 8 O 4 , FM 132.11) to give a pH of 4.40 when diluted to 250 mL?
-
Calculate the pH of a 0.010 M solution of each amino acid in the form drawn here. H2N NH2 NH2 NH C=0 CH2 CH2 S CH, CH, CH2 CH2 (a) H;NCHCO, (b) H&NCHCO, (c) H,NCHCO, Glutamine Cysteine Arginine
-
(a) Draw the structure of the predominant form (principal species) of 1,3-dihydroxybenzene at pH 9.00 and at pH 11.00. (b) What is the second most prominent species at each pH? (c) Calculate the...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


