Effective mass. For some materials, the band structure of the conduction band around k = 0 can
Question:
Effective mass. For some materials, the band structure of the conduction band around k = 0 can be represented by 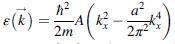
What is the effective mass of a free electron under these conditions? On the figure, name the different bands and point out which one of the two in the lower band has the higher effective mass. 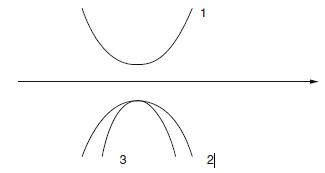
Transcribed Image Text:
a? (k -AK 2n2" !! 2m
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
Ekh2mAka2nk Effective mass q electron is given by MhdEdk Since h2m is constant con...View the full answer

Answered By

Collins Bett
i work in Science and engineer,physics,mathematics,chemistry and etc.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
A satellite single-axis attitude control system can be represented by the block diagram in Figure CP2.5. The variables k, a, and b are controller parameters, and J is the spacecraft moment of...
-
A linear dynamical system can be represented by the equations Dx / dt = A(t)x(t) + B(t)u(t), y(t) = C(t)x(t) + D(t)u(t), where A is an n n variable matrix, B is an n r variable matrix, C is an m n...
-
The polarization of a carbonyl group can be represented by a pair of resonance structures: Cyclopropenone and cycloheptatrienone are more stable than anticipated. Cyclopentadienone, however, is...
-
Trade promotions refer to offers made to Group of answer choices channel members non-profit organizations businesses consumers Question 2 Consumer promotions refer to offers made to Group of answer...
-
Chris Cho is a veterinary surgeon, specializing in pigs. His customers are pig farmers, large and small, throughout the province. To support his practice, he also operates All Creatures Veterinary...
-
A 1.00-kg block of aluminum is heated at atmospheric pressure so that its temperature increases from 22.0C to 40.0C. Find (a) The work done on the aluminum, (b) The energy added to it by heat and (c)...
-
7. Explain the circumstances under which fair-value hedge accounting should be used and when cash-flow hedge accounting should be used.
-
Lawrence loaned money to Moore, who died without repaying the loan. Lawrence claimed that when he mentioned the matter to Moores widow, she promised to pay the debt. She did not pay it, and Lawrence...
-
The primary reason a company wants to go public is what? O Greater visibility Access to capital Easier recruitment of talent O Maintenance of confidentiality of financial data
-
For each of the following, review the data in the images below and identify the (1) delimiter and (2) the qualifier (if applicable). Is an asset ID a good data field to use as a unique identifier in...
-
A single electron is placed at k = 0 in an otherwise empty band of a bcc solid. The energy versus k relation of the band is given by: At t = 0, a uniform electric field E is applied in the x-axis...
-
Calculate the coordinates of the high-symmetry point U in Fig. 5.15. W L. K
-
Randomly selecting a movie with a rating of R Randomly selecting a movie with a rating of four stars Determine whether the two events are disjoint for a single trial.
-
n1 = 20, n2 = 25, S = 607, H1: 1 2. In Exercises 710, compute S, S, and the value of the test statistic z. Then find the P-value for the specified alternate hypothesis and values of n1, n2, and S.
-
To determine whether traffic levels differ between the morning and evening rush hours, a traffic engineer counted the number of cars passing through a certain intersection during five-minute periods...
-
Macon Timber Company established a \(\$ 150\) petty cash fund on January 1, 2012. Required a. Is the establishment of the petty cash fund an asset source, use, or exchange transaction? b. Record the...
-
Following is a bank reconciliation for Holt's Sandwich Shop for May 31, 2012: Because of limited funds, Holt's employed only one accountant who was responsible for receiving cash, recording receipts...
-
For each of the following situations, fill in the blank with FIFO, LIFO, or weighted average. a. b. c. d. e. f. would produce the highest amount of net income in an inflationary environment. would...
-
Why should persons who pursue careers in business have a basic understanding of finance even if their jobs are in areas other than finance, such as marketing or information systems?
-
Pearson Education, a publisher of college textbooks, would like to know if students prefer traditional textbooks or digital textbooks. A random sample of students was asked their preference and the...
-
Fractional composition in a triprotic system. For a triprotic system, the fractional composition equations are where D = [H + ] 3 + K 1 [H + ] 2 + K1K 2 [H + ] + K 1 K 2 K 3 . Use these equations to...
-
Explain what is wrong with the following statement: At its isoelectric point, the charge on all molecules of a particular protein is 0.
-
Calculate the pH at each of the following points in the titration of 50.00 mL of 0.010 0 M NaOH with 0.100 M HCl. Volume of acid added: 0.00, 1.00, 2.00, 3.00, 4.00, 4.50, 4.90, 4.99, 5.00, 5.01,...
-
FINANCIAL STATEMENT ANALYSIS INSTRUCTIONS 1. PREPARE RATIO ANALYSIS REPORT ( word file) Format 1. Introduction 2. Importance of Financial Statements 3. Importance of Financial statement analysis and...
-
Let us assume that Europe is in recession, China's economy is slowing down, and the US economy is growing at 1-2%. Use these assumptions to invest in 4 ETFs (electronically traded funds). The 4 ETFs...
-
A section 83(b) election creates ordinary income at the time of the grant. Ture or False

Study smarter with the SolutionInn App


