The interaction energy between Na + and Cl - ions in the NaCl crystal can be written
Question:
The interaction energy between Na+ and Cl- ions in the NaCl crystal can be written as
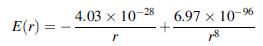
where the energy is given in joules per ion pair and the interionic separation r is in meters. The numerator unit of the first term is J-m and the second term is J-m8. Calculate the binding energy and the equilibrium separation between the Na+ and Cl- ions.
Transcribed Image Text:
E(r) = 4.03 x 10-28 6.97 x 10-9%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
The interaction energy between Na and Cl ions in the NaCl crystal can be written as Er 403 x 10...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The accompanying graph depicts the interaction energy between two water molecules situated so that their dipole moments are parallel and pointing in the same direction. Sketch an approximate curve...
-
The distance between the K+ and Cl ions in KCl is 2.80 1010 m. Calculate the energy required to separate the two ions to an infinite distance apart, assuming them to be point charges initially at...
-
The distance between the Li+ and Cl ions in LiCl is 0.257 nm. Use this and the molecular mass of LiCl (42.4 g/mol) to compute the density of LiCl.
-
Grimm Company has 2,400,000 shares of common stock outstanding on December 31, 2014. An additional 150,000 shares of common stock were issued on July 1, 2015, and 300,000 more on October 1, 2015. On...
-
Warren Buffett has been a very successful investor. In 2008 Luisa Kroll reported that Buffett topped Forbes Magazine's list of the world's richest people with a fortune estimated to be worth $62...
-
Where are fossils found?
-
Consider the data from Problem 14-10. The human resources department requires that non-dimensional scaling be applied to make your decision. Rate the individual attributes using Equations (14-1) and...
-
The output of a regression of overhead costs on direct labor costs per month follows: Regression Results Equation:...
-
Asset source transactions include obtaining cash from the issue of common stock purchasing supplies on account providing services on account purchasing land for cash
-
A steel block with a density of 7800 kg/m 3 is suspended from a string in a beaker of water so that the block is completely submerged but not resting on the bottom. The block is a cube with sides of...
-
Calculate the total coulombic potential energy of a Na + in a NaCl crystal by considering only up to the fourth nearest neighbors of Na +. The coulombic potential energy for two ions of opposite...
-
Consider the van der Waals bonding in solid argon. The potential energy as a function of interatomic separation can generally be modeled by the Lennard-Jones 612 potential energy curve, that is, E(r)...
-
Write the code to find the square root of the number stored in the first row, third column in a two-dimensional double array named mathNumbers. Display the result on the screen.
-
IFRS Financial Statements Thomson Reuters is a global information company created by the 2008 merger of the Thomson Corporation, a Canadian company, with the Reuters Company, a United Kingdom-based...
-
Burgess Services Co. experienced the following events in 2011: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
In Exercises 13 and 14, use the box-and-whisker plot to identify the five-number summary. 0 2 5 8 10 ++ ++ 0 1 2 3 4 5 6 7 8 9 10 11
-
In a test of the effect of dampness on electrical connections, 80 electrical connections were tested under damp conditions and 130 were tested under dry conditions. Twenty of the damp connections...
-
Zelta Ltd. is a medium-size company involved in providing a range of specialized products and services for the aerospace industry. Just over a year ago, external consultants undertook a major review...
-
Boat Emporium (BE) must raise $225 million. To do so, BE expects to issue new common stock. BEs Investment banker will charge issuing costs equal to 10 percent of the total amount issued. If the...
-
Banner Company acquires an 80% interest in Roller Company for $640,000 cash on January 1, 2013. The NCI has a fair value of $160,000. Any excess of cost over book value is attributed to goodwill. To...
-
We have a 37.0 ( 0.5) wt% HCl solution with a density of 1.18 ( 0.01) g/mL. To deliver 0.050 0 mol of HCl requires 4.18 mL of solution. If the uncertainty that can be tolerated in 0.050 0 mol is 2%,...
-
Compute the molecular mass and its standard uncertainty for NH 3 . What is the percent relative uncertainty in molecular mass?
-
How many significant figures are there in the following numbers? (a) 1.903 0 (b) 0.039 10 (c) 1.40 10 4
-
Describe how the following affect the valuation of PPE. a) Cash Discounts b) Deferred Payment Contracts
-
Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new products for a five - year period. His annual pay raises are determined by his division s...
-
Consider a 5 year debt with a 15% coupon rate paid semi-annually, redeemable at Php1,000 par. The bond is selling at 90%. The flotation cost is Php50 per bind. The firm's tax bracket is 30%.

Study smarter with the SolutionInn App


