A film of ethanol at 20C has a surface tension of 22.3 mN/m and is maintained on
Question:
A film of ethanol at 20◦C has a surface tension of 22.3 mN/m and is maintained on a wire frame, as shown in Fig. P3.202. Consider the film with two surfaces as a control mass and find the work done when the wire is moved 10 mm to make the film 20 × 40 mm.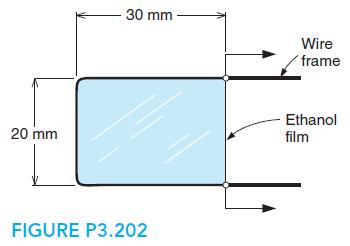
30 mm Wire frame -Ethanol 20 mm film FIGURE P3.202
Step by Step Answer:
To find the work done when the wire is moved to make the film 20 x 40 mm we can u...View the full answer



Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Related Video
The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface of a glass of water do not have other water molecules on all sides of them and consequently, they cohere more strongly to those directly associated with them (in this case, next to and below them, but not above). is not really true that a \\\"skin\\\" forms on the water surface; the stronger cohesion between the water molecules as opposed to the attraction of the water molecules to the air makes it more difficult to move an object through the surface than to move it when it is completely submersed
Students also viewed these Sciences questions
-
A film of ethanol at 20C has a surface tension of 22.3 mN/m and is maintained on a wire frame as shown in Fig. P4.73. Consider the film with two surfaces as a control mass and find the work done when...
-
Two tanks are connected together as shown in Fig. P3.49, both containing water. Tank A is at 30 lbf/in.2, v = 8 ft3/lbm, V = 40 ft3 and tank B contains 8 lbm at 80 lbf/in. 2, 750 F. The valve is now...
-
As shown in fig 1.7, a manometer is attached to a tank of gas in which the pressure is 104.0 kPa. The manometer liquid is mercury, with a density of 13.59 g/cm3. If g = 9.81 m/s2 and the atmospheric...
-
If $4000 is deposited into an account paying 3% interest compounded annually and at the same time $2000 is deposited into an account paying 5% interest compounded annually, after how long will the...
-
Explain the various types of endorsements for checks?
-
The capacitor shown in Fig. 4-36 (P4.54) consists of two parallel dielectric layers. We wish to use energy considerations to show that the equivalent capacitance of the overall capacitor, C, is equal...
-
Study of analysts forecasts. The Journal of Accounting Research (March 2008) published a study on relationship incentives and degree of optimism among analysts forecasts. Participants were analysts...
-
Suppose Leonard Krauss places an order to buy 100 shares of Google. Explain how the order will be processed if its a market order. Would it make any difference if it had been a limit order? Explain.
-
The cash payments of The Aristocrats Jewels, a retail business, for June and the general ledger accounts used to record these transactions appear below. GENERAL LEDGER ACCOUNTS 1 0 1 Cash $ 4 6 , 7 4...
-
The following information is taken from the accounts of Latta Company. The entries in the T-accounts are summaries of the transactions that affected those accounts during the year. The overhead that...
-
The piston/cylinder arrangement in Fig. P3.181 contains 10 g ammonia at 20C with a volume of 1 L. There are some stops, so if the piston is at the stops, the volume is 1.4 L. The ammonia is now...
-
A sheet of rubber is stretched out over a ring of radius 0.25 m. I pour liquid water at 20C on it, as in Fig. P3.205, so that the rubber forms a half sphere (cup). Neglect the rubber mass and find...
-
For intercompany transfers of depreciable fixed assets at a gain or loss, how and when does realization of intercompany gains and losses occur?
-
Can anyone explain me how to calculate the ROI using the HISTORICAL COST NBV, the formula my instructor wants me to use is ADJ CF - HIST DEP /ASSETTOTAL - ACC DEP. And for the ROI of CURRENT COST NBV...
-
Consider the circuit to the right 3. If the total voltage supply in the circuit is 120V, and each resistor has a resistance of 400, what will the current read on each ammeter? |1= 12= 3 = 4. What...
-
1. The theory predicts the proportion of beans, in the four groups A, B, C and D should be 9:3:3:1. In an experiment among 1600 beans, the numbers in the four groups were 882, 313, 287 and 118. Does...
-
Would you recommend criminal charges in this case ( the screenshots below) and, if so, exactly which statutes against which person? Explain your reasoning (how the elements of the crime are met or...
-
check if each transaction is placed in the right place in each of the reports below and if there are any other mistakes in the different accounts after the first image which is a description of the...
-
What are the advantages of nonstatistical sampling over statistical sampling?
-
Consider model (9.18). What is the effect on the model parameter estimates, their standard errors, and the goodness-of-fit statistics when (a) The times at risk are doubled, but the numbers of deaths...
-
An insulated cylinder fitted with a piston contains 0.1 kg of water at 100C, 90% quality. The piston is moved, compressing the water until it reaches a pressure of 1.2 MPa. How much work is required...
-
Compression and heat transfer brings R-134a in a piston/cylinder from 500 kPa, 50oC to saturated vapor in an isothermal process. Find the specific heat transfer and the specific work.
-
One kilogram of water at 300C expands against a piston in a cylinder until it reaches ambient pressure, 100 kPa, at which point the water has a quality of 90.2%. It may be assumed that the expansion...
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


