A reversible process in a piston/cylinder is shown in Fig. P6.7. Indicate the storage change u 2
Question:
A reversible process in a piston/cylinder is shown in Fig. P6.7. Indicate the storage change u2 − u1 and transfers 1w2 and 1q2 as positive, zero, or negative.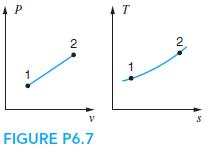
Transcribed Image Text:
2. FIGURE P6.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Question Question Description A reversible process in a ...View the full answer

Answered By

Tarique Anwar Khan
I am teaching in an engineering college from last 7 years.
I was in Shivneri polytechnic Junnar from 2013 to 2014. then i have joined to MMANTC, Malegaon as an assistant professor in department of mechanical engineering in 2014 and currently working there.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A reversible process in a steady flow of air with negligible kinetic and potential energy changes is shown in Fig. P7.4. Indicate the change h e h i and transfers w and q as positive, zero, or...
-
A closed gaseous system undergoes a reversible process in which 30 kJ of heat is rejected and the volume changes from 0.14 m 3 to 0.055 m 3 The pressure is constant at 150 kPa. Determine (a) the...
-
A reversible process in a piston/cylinder is shown in Fig. P6.8. Indicate the storage change u 2 u 1 and transfers 1w 2 and 1q 2 as positive, zero, or negative. P 1 FIGURE P6.8 2. 2.
-
O 00:29:33 4. Let an be a convergent series and b, be a soquence such that 0 <2+ a, < b Which of the following statements are true? 10 I) The convergence or divergence of cannot be concluded. Im (2+...
-
You listen to a debate between two politicians discussing the economic health of the United States. One politician says that the average income of all workers in the United States is $72,235; the...
-
A uniform sphere of radius r and mass m is placed with no initial velocity on a belt that moves to the right with a constant velocity v1. Denoting by k the coefficient of kinetic friction between the...
-
The first entry in the standard-layout cash flow statement is cash flow from operating activities. How is this figure different from the net profit before interest and tax for the same period?
-
Franklin Co. has experienced gross profit ratios for 2010, 2009, and 2008 of 33%, 30%, and 31%, respectively. On April 3, 2011, the firms plant and all of its inventory were destroyed by a tornado....
-
What is the amount of annual property taxes due for a property that qualifies for a homestead exemption of $35,000 in a jurisdiction that imposes a total millage rate of 47.5 mills and has a market...
-
Follow the instructions to work through the calculation of cost per billable test for a drug screen composed of five independently measurable assays, using the following information: . Instrument was...
-
Liquid water at 20C, 100 kPa is compressed in a piston/cylinder without any heat transfer to a pressure of 200 kPa. How do the properties (T, v, u, and s) change (increase, stay about the same, or...
-
Air at 290 K, 100 kPa in a rigid box is heated to 325 K. How do the properties (P, v, u, and s) change (increase, stay about the same, or decrease)?
-
How are the four fundamental aspects of organizational structure related to one another? Why might it be difficult to change one fundamental without also changing the others?
-
Financial strength can be defined as the capacity to produce enough cash flows and earnings to pay creditors, investors, and other debts, as well as to cover expenses. Even though sales by themselves...
-
The RMS Titanic was the most technologically advanced liner in the world in the year 1912. At 11:40pm or Sunday, April 14 of that year, the Titanic struck an iceberg and sank in less than three...
-
1. A management consultant is hired by a manufacturing firm to determine the best site for its next production facility. The consultant has had several meetings with the company's senior executives...
-
The figure below shows that a pump is used to transfer water from a reservoir at ground level to a storage take that is elevated. The pump is located 10 ft above the water surface of the reservoir...
-
P6-3 (Algo) Comparing and Contrasting the Effects of Inventory Costing Methods on Financial Statement Elements LO6-2, 6-3 Neverstop Corporation sells item A as part of its product line. Information...
-
Twin primes are a pair of prime numbers that differ by 2. For example, 3 and 5 are twin primes, 5 and 7 are twin primes, and 11 and 13 are twin primes. Write a program to find all twin primes less...
-
Avatar Financials, Inc., located on Madison Avenue, New York City, is a company that provides financial advice to individuals and small- to mid-sized businesses. Its primary operations are in wealth...
-
At 298.15 K, G f (HCOOH, g) = -351.0 kJ mol -1 and G f (HCOOH, l) 361.4 kJ mol -1 . Calculate the vapor pressure of water at this temperature.
-
In this problem, you will calculate the differences in the chemical potentials of ice and super cooled water, and of steam and superheated water, all at 1 atm pressure shown schematically in Figure...
-
Calculate the vapor pressure of a droplet of benzene of radius 1.25 10 8 m at 38.0C in equilibrium with its vapor. Use the tabulated value of the density and the surface tension at 298 K from...
-
Bought an old van for 4000 from Peters promising to pay laterwhat is the transactions
-
Company has a following trade credit policy 1/10 N45. If you can borrow from a bank at 9,5% annual rate, would it be beneficial to borrow money and pay off invoices earlier?
-
Given the following exchange rates, which of the multiple-choice choices represents a potentially profitable inter-market arbitrage opportunity? 129.87/$1.1226/$0.00864/ 114.96/ B $0.8908/ (C)...

Study smarter with the SolutionInn App


