Liquid acetylene, C 2 H 2 , is stored in a high pressure storage tank at ambient
Question:
Liquid acetylene, C2H2, is stored in a high pressure storage tank at ambient temperature, 25â¦C. The liquid is fed to an insulated combustor/steam boiler at a steady rate of 1 kg/s, along with 140% theoretical oxygen, O2, which enters at 500 K, as shown in Fig. P13.162. The combustion products exit the unit at 500 kPa, 350 K.
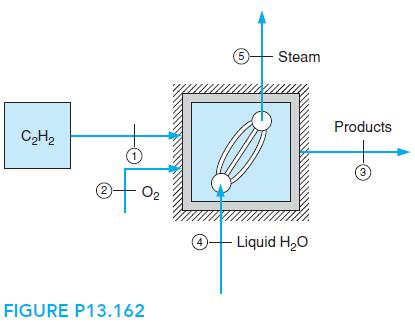
Liquid water enters the boiler at 10â¦C, at the rate of 15 kg/s, and superheated steam exits at 200 kPa.
a. Calculate the absolute entropy, per kmol, of liquid acetylene at the storage tank state.
b. Determine the phase(s) of the combustion products exiting the combustor boiler unit and the amount of each if more than one.
c. Determine the temperature of the steam at the boiler exit.
Step by Step Answer:

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag





