Prove that the two relations for changes in s, Eqs. 6.16 and 6.17, are equivalent once we
Question:
Prove that the two relations for changes in s, Eqs. 6.16 and 6.17, are equivalent once we assume constant specific heat.
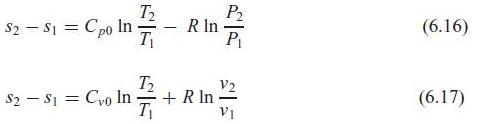
Transcribed Image Text:
P2 R In P1 T2 $2 - S1 = Cpo I (6.16) T2 V2 + R In T S2 - S1 = Cyo In (6.17) V1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
To prove that Eqs 616 and 617 are equivalent when we assume constant specific heat we start with Eq ...View the full answer

Answered By

Anik Kumar Bosu
I am expert in Maths, Physics, Chemistry and Biology. I also teach student from 3 years. Students becomes satisfied by my teaching. So, do not worry.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Prove that the two relations for entropy change of ideal gases under the constant-specific-heat assumption (Eqs. 733 and 734) are equivalent.
-
Prove that the two equations for E in the example starting on page B-7 are equivalent by using DeMorgans theorems and the axioms shown on page B-7.
-
Question 3 Prove that the two shaded areas in the graphs below are equal. y y 1+ A 1 f(x)= X 1+x4 2 0.5 -0.5 B 1 g(x) = 2(1+x) 2 3 4 X
-
a) Analysts attempt to value ordinary shares, in order to determine if market values are correct. Identify and describe the more common methods of valuation. Include a detailed explanation of the...
-
For each of the following examples, indicate whether it involves the use of descriptive or inferential statistics. Justify your answer. a. The number of unemployed people in the United States b....
-
Show that u' = p, p' = u + b, b' = p.
-
Assuming a 10 percent annual discount rate, which system should EAMO purchase? Cost Estimates for EOP and PMS Year EOP ($) PMS ($) 0 (the current year) 1,500,000 750,000 1 100,000 300,000 2 100,000...
-
The SEC Form 10-K on NIKE is reproduced in Appendix C. REQUIRED: Review the NIKE SEC Form 10-K, answer the following question: a. What percentage of total assets do property, plant, and equipment and...
-
15. Last year Janet purchased a $1,000 face value corporate bond with an 7% annual coupon rate and a 30-year maturity. At the time of the purchase, it had an expected yield to maturity of 13.46%. If...
-
The Mead Company uses a perpetual inventory system and engaged in the following transactions during the month of May: Date Transaction_______________________________________________ May 1 Made cash...
-
Three kilograms of air is in a piston/cylinder keeping constant pressure at 27C, 300 kPa. It is now heated to 500 K. Plot the process path in a Ts diagram and find the heat transfer in the process.
-
A closed rigid container is filled with 1.5 kg water at 100 kPa, 55C, 1 kg of stainless steel and 0.5 kg of polyvinyl chloride, both at 20Cand 0.1 kg of hot air at 400 K, 100 kPa. It is now left...
-
How does Level 5 leadership differ from the concept of servant leadership? Do you believe anyone has the potential to become a Level 5 leader? Discuss.
-
Part 1 - Financial Statement Analysis Income Statement Kirks Family Restaurant December 31, 2018 Sales 480,000 Interest revenue 15,000 Total Revenue 495,000 Cost of goods sold 200,000 Gross Margin...
-
Find the most general value of satisfying tan 0 = -3.
-
(i) Undercasting of the debit side of Bank column. 70 (ii) Cheques issued but not presented for payment till 01-01-2011. 1,450 (1,520) 2,179 Bank Balance as per Pass Book as on 1-1-2011. Different...
-
Determine the stiffness matrix K for the truss. Take A = 0.0015 m^2 and E = 200 GPa for each member. Please show the step-by-step solution. 5 410 9 3 5 7 7 8 A4 Tesol242 3 2 4 4 5 6 2 4 m 4 m 20 kN...
-
Probability Mr Pandazis Practice Questions for Test #1 Math 241 1. Define a sample space S for the following experiment. Toss a coin three times and record the outcome for each toss. 2. A card is...
-
Write a program that reads an unspecified number of integers, determines how many positive and negative values have been read, and computes the total and average of the input values (not counting...
-
Assume that a trial balance is prepared with an account balance of $21,360 listed as $21,630 and an account balance of $1,500 listed as $15,000. Identify the transposition and the slide.
-
Calculate the mean ionic molality and mean ionic activity of a 0.105 m K 3 PO 4 solution for which the mean ionic activity coefficient is 0.225.
-
The equilibrium constant for the hydrolysis of dimethylamine, (CH 3 ) 2 NH(aq) + H 2 O(aq) CH 3 NH 3 + (aq) + OH (aq) Is 5.12 10 4 . Calculate the extent of hydrolysis for a. A 0.210 m solution of...
-
Consider the cell Pt(s)|H 2 (g,1atm)|H + (aq, a = 1)|Fe 3+ (aq),Fe 2+ (aq)|Pt(s) given that Fe 3+ (aq) + e Fe 2+ (aq) and E = 0.771V. a. If the cell potential is 0.712V, what is the ratio of Fe2+...
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


