The cogenerating power plant shown in Problem 9.80 burns 170 kg/s air with natural gas, CH 4
Question:
The cogenerating power plant shown in Problem 9.80 burns 170 kg/s air with natural gas, CH4. The setup is shown in Fig. P13.205, where a fraction of the air flow out of the compressor with pressure ratio 15.8:1 is used to preheat the feedwater in the steam cycle. The fuel flowrate is 3.2 kg/s. Analyze the system, determining the total heat transfer to the steam cycle from the turbine exhaust gases, the heat transfer in the preheater, and the gas turbine inlet temperature.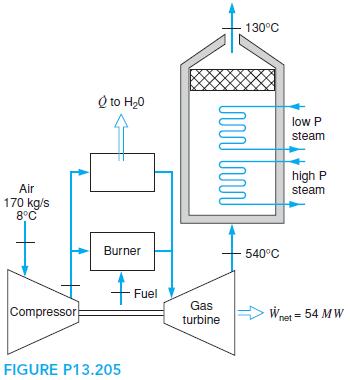
Transcribed Image Text:
130°C Q to H20 low P steam high P Air steam 170 kg/s 8°C Burner 540°C Fuel Gas Compressor Wnet = 54 MW turbine FIGURE P13.205
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
In order to analyze the system and determine the total heat transfer to the steam cycle from the turbine exhaust gases the heat transfer in the prehea...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
The simple steam power plant shown in Problem 6.39 has a turbine with given inlet and exit states. Find the availability at the turbine exit, state 6. Find the second law efficiency for the turbine,...
-
The simple steam power plant shown in Problem 6.99 has a turbine with given inlet and exit states. Find the availability at the turbine exit, state 6. Find the second law efficiency for the turbine,...
-
The asterisk in the structure shown in Problem 9 indicates the site where the base is attached via a two-carbon linker. Compare the overall backbone length for a PNA and a DNA molecule and explain...
-
As mentioned in Section 5.6, Sainte-Venants principle will allow particular boundary conditions to be replaced by their statically equivalent resultant. For problems (b), (c), (d),and (f) in Exercise...
-
Solve previous Problem 25.12 except that the current inventory on hand and on order for S3, C6, and M6 is as follows: for S3, inventory on hand is 2 units and quantity on order is zero; for C6,...
-
Four 9.5-kg spheres are located at the corners of a square of side 0.60m. Calculate the magnitude and direction of the total gravitational force exerted on one sphere by the other three.
-
Emotional state: Angry Neutral Guilt
-
Speedy Auto Wash is contemplating the purchase of a new high-speed washer to replace the existing washer. The existing washer was purchased two years ago at an installed cost of $120,000; it was...
-
in the audit o Pnco Cu c Company o he year onde Septomoor 0 he differences uncovererd in the ronfimmatian ne a d or sot a t abl ms ate ont o $15 000 at an A IA O 15% A PP sam c o 100 was s oct d from...
-
1. Of all the UPL conduct listed in the case, could any of it be performed by a legitimate paralegal? 2. If there was an attorney involved with WTP/Sarasota, how was UPL committed? 3. After this...
-
Study the coal gasification process that will produce methane, CH 4 , or methanol, CH 3 OH. What is involved in such a process? Compare the heating values of the gas products with those of the...
-
A car that runs on natural gas has it stored in a heavy tank with a maximum pressure of 3600 psi (25 MPa). Size the tank for a range of 300 miles (500 km), assuming a car engine that has a 30%...
-
State whether each of the following statements is true or false. Justify your answer in each case. (a) Sulfuric acid is a monoprotic acid. (b) HCl is a weak acid. (c) Methanol is a base.
-
The financial statements for the Columbia Sportswear Company can be found in Appendix A, and Under Armour, Inc.'s financial statements can be found in Appendix B at the end of this book. Required a....
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts to income summary. The balance of \(\$ 8,500\) in the retained earnings account is from the...
-
Adjusting Entries The following selected accounts appear in the Birch Company's unadjusted trial balance as of December 31, the end of the fiscal year (all accounts have normal balances): Required...
-
Closing Entries Use the information provided in E3-5A to prepare journal entries to close the accounts using the Income Summary account. After these entries are posted, what is the balance in the...
-
Ceva, Inc. manufactures and services jet engines for air carriers. The engines cost \($10\) to \($40\) million each, depending on the specifications and plane. A 10-year service contract for a single...
-
Calculate the total 2017 tax liability for a surviving spouse with one dependent child with a gross income of $46,250, no salary reductions for employer-provided benefits, and no itemized deduction.
-
Could the owner of a business prepare a statement of financial position on 9 December or 23 June or today?
-
Liquid n-butane at T0, is sprayed into a gas turbine with primary air flowing at 1.0 MPa, 400 K in a stoichiometric ratio. After complete combustion, the products are at the adiabatic flame...
-
Butane gas at 25C is mixed with 150% theoretical air at 600 K and is burned in an adiabatic steady flow combustor. What is the temperature of the products exiting the combustor?
-
Natural gas, we assume methane, is burned with 200% theoretical air and the reactants are supplied as gases at the reference temperature and pressure. The products are flowing through a heat...
-
American Food Services, Incorporated leased a packaging machine from Barton and Barton Corporation. Barton and Barton completed construction of the machine on January 1 , 2 0 2 4 . The lease...
-
Which of the following statements is true? Financial measures tend to be lag indicators that report on the results of past actions. LA profit center is responsible for generating revenue, but it is...
-
Andretti Company has a single product called a Dak. The company normally produces and sells 8 0 , 0 0 0 Daks each year at a selling price of $ 5 6 per unit. The company s unit costs at this level of...

Study smarter with the SolutionInn App


