Water in a piston/cylinder (Fig. P3.174) is at 101 kPa, 25C, and mass 0.5 kg. The piston
Question:
Water in a piston/cylinder (Fig. P3.174) is at 101 kPa, 25◦C, and mass 0.5 kg. The piston rests on some stops, and the pressure should be 1000 kPa to float the piston. We now heat the water, so the piston just reaches the end of the cylinder. Find the total heat transfer.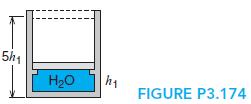
Transcribed Image Text:
5h1 H20 FIGURE P3.174
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
Assuming no phase change in water httpwww1lsb...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Water is in a piston cylinder maintaining constant P at 700 kPa, quality 90% with a volume of 0.1 m3. A heater is turned on heating the water with 2.5 kW. What is the rate of mass (kg/s) vaporizing?
-
Water is in a piston cylinder maintaining constant P at 330 F, quality 90% with a volume of 4 ft3. A heater is turned on heating the water with 10 000 Btu/h. What is the elapsed time to vaporize all...
-
10 kg of water in a piston cylinder arrangement exists as saturated liquid/vapor at 100 kPa, with a quality of 50%. It is now heated so the volume triples. The mass of the piston is such that a...
-
Air temperature usually decreases with increasing altitude. However, during the winter, thanks to a phenomenon called thermal inversion, the temperature of air warmed by the sun in mountains above a...
-
Bottletree Bakery and Card Shop ordered an equal number of 12 different cards. If a total of 60 cards were ordered, how many of each type of card were ordered?
-
A laser beam traveling through fog was observed to have an intensity of 1 (W/m2) at a distance of 2 m from the laser gun and an intensity of 0.2 (W/m2) at a distance of 3 m. Given that the intensity...
-
Biology class. The students in a biology class are categorizing organisms that can swim and breath using fins, such as fishes, sharks, and whales, and those that can swim but have no fins, such as...
-
Many retail stores offer to match or beat the price offered by a rival store. Explain why firms that belong to a cartel might make this offer.
-
Why the Chip Shortage Is So Hard to vercome By Eun-Young Jeong in Seoul and Dan Strumpf in Hong Kong
-
You may refer to the opening story of Tony and Suzie and their decision to start Great Adventures in AP 1-1. More of their story and the first set of transactions for the company in July are...
-
A vertical cylinder fitted with a piston contains 5 kg of R-410a at 10C, as shown in Fig. P3.173. Heat is transferred to the system, causing the piston to rise until it reaches a set of stops, at...
-
The piston/cylinder in Fig. P3.179 contains 0.1 kg R-410a at 600 kPa, 60C. It is now cooled, so the volume is reduced to half of the initial volume. The piston has upper stops mounted, and the piston...
-
The EMI Group The shareholders equity section of the balance sheet of The EMI Group, a company based in the United Kingdom, is reproduced below. Review this information and answer the questions...
-
A release has been planned with 5 sprints. The team, for the sake of convenience, has decided to keep the sprint duration open. Depending on how much they commit and achieve, they decide to wrap up...
-
Task 3: Reach-truck management 3 Explain why battery-powered reach truck activities at PAPFS are unsatisfactory. Note: You should support your answer, where applicable, using relevant information...
-
Exercise 6: Black Pearl, Inc., sells a single product. The company's most recent income statement is given below. Sales $50,000 Less variable expenses Contribution margin Less fixed expenses Net...
-
Your maths problem x+3x-3
-
Spencer is a 10-year-old boy who has been living in a family-style therapeutic group home for one year. He was removed from his mother's care due to neglect from her drug use and the resulting legal...
-
Audit sampling is defined as a situation where: a. The auditor tests a subset of the population to draw a conclusion about a subset of the population. b. The auditor screens 100% of the population to...
-
If M = 7, s = 2, and X = 9.5, what is z?
-
A cylinder fitted with a piston contains 0.5 kg of R-134a at 60C, with a quality of 50 percent. The R-134a now expands in an internally reversible polytropic process to ambient temperature, 20C at...
-
A cylinder with a linear spring-loaded piston contains carbon dioxide gas at 2 MPa with a volume of 50 L. The device is of aluminum and has a mass of 4 kg. Everything (Al and gas) is initially at...
-
A vertical cylinder/piston contains R22 at 20C, 70% quality, and the volume is 50 L, shown in Fig. P8.138. This cylinder is brought into a 20C room, and an electric current of 10 A is passed through...
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.
-
You wish to buy a car today for $35,000. You plan to put 10% down and finance the rest at 5.20% p.a. for six years. You will make equal monthly payments of $_______.

Study smarter with the SolutionInn App


