A 0.0240 mol sample of N 2 O 4 (g) is allowed to come to equilibrium with
Question:
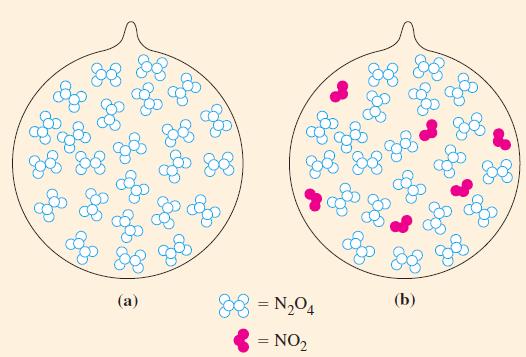
A 0.0240 mol sample of N2O4(g) is allowed to come to equilibrium with NO2(g) in a 0.372 L flask at 25 °C. Calculate the amount of N2O4 present at equilibrium (Fig. 15-9).
![]()
Figure 15-9
Transcribed Image Text:
N₂O4(g) 2 NO₂(g) Kc = 4.61 × 103 at 25 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Analyze We need to determine the amount of N 2 O 4 that dissociates to establish equilibrium For the ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) If 0.150 mol H 2 (g) and 0.200 mol I 2 (g) are introduced into a 15.0 L flask at 445 C and allowed to come to equilibrium, how many moles of HI(g) will be present? (B) Suppose the equilibrium...
-
A 1.100 L flask at 25 C and 1.00 atm pressure contains CO 2 (g) in contact with 100.0 mL of a saturated aqueous solution in which [CO 2 (aq)] = 3.29 x 10 -2 M. (a) What is the value of K c at 25 C...
-
A.0.50 mole sample of N2O4(g) is allowed to come to equilibrium with NO2(g) in a 0.750L flask. At equilibrium , 0.42mol N2O4 remains. Calculate the equilibrium concentrations of N2O4 and NO2 and...
-
The following trial balance is taken from the General Fund of the City of Jennings for the year ending December 31, 2017. Prepare a condensed statement of revenues, expenditures, and other changes in...
-
Valley Corporation is attempting to select the best of a group of independent projects competing for the firms fixed capital budget of $4.5 million. The firm recognizes that any unused portion of...
-
Western Sound Studios records and masters audio tapes of popular artists in live concerts. The performers use the tapes to prepare live albums, CDs, and MP3s. The following account balances were...
-
6 In measuring the results of social media, what are the (a) advantages and (b) disadvantages of performance measures linked directly to revenues versus costs?
-
How is an organization like an iceberg? Use the iceberg metaphor to describe the field of organizational behavior.
-
The Saint Lucia Blood Bank, a private charity partly supported by government grants, is located on the Caribbean island of Saint Lucia The blood bank has just finished its operations for September,...
-
Given the equilibrium constant values 1 N2(g) + O2(g) = NO(g) K = 2.7 10-18 2 NO2(g) Kc = 4.6 x 10- Kc = 4.1 10- N2O4(g) N2(g) + O2(g) NO(g) N2(g) + O2(g) 2 Determine a value of K, for the...
-
Determine K c for the reaction 2 N2(g) + O2(g) + Br2(g) NOBr(g) from the following information (at 298 K). 2 NO(g) 1 Br(g) NO(g) + 2 N2(g) + O(g) K = 2.1 10 NOBr(g) K = 1.4
-
In Problem perform a mental calculation to find the answer and include the correct units. Find the total area enclosed by 4 non-overlapping rectangles, if each rectangle has width 2 meters and the...
-
IFRS Financial Statements Thomson Reuters is a global information company created by the 2008 merger of the Thomson Corporation, a Canadian company, with the Reuters Company, a United Kingdom-based...
-
Burgess Services Co. experienced the following events in 2011: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
In Exercises 13 and 14, use the box-and-whisker plot to identify the five-number summary. 0 2 5 8 10 ++ ++ 0 1 2 3 4 5 6 7 8 9 10 11
-
In a test of the effect of dampness on electrical connections, 80 electrical connections were tested under damp conditions and 130 were tested under dry conditions. Twenty of the damp connections...
-
Zelta Ltd. is a medium-size company involved in providing a range of specialized products and services for the aerospace industry. Just over a year ago, external consultants undertook a major review...
-
Douglas Corp. has experienced tremendous sales growth this year, but it is always short of cash. What is one explanation for this occurrence?
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
The consulting firm of Tilton and Henderson accumulates costs associated with individual cases, using a job order cost system. The following transactions occurred during June: June 4. Charged 600...
-
The Ad Guys Inc. provides advertising services for clients across the nation. The Ad Guys is presently working on four projects, each for a different client. The Ad Guys accumulates costs for each...
-
Keltner Co. uses a job order cost system. The following data summarize the operations related to production for November: a. Materials purchased on account, $350,000. b. Materials requisitioned,...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


