Determine K c for the reaction 2 N2(g) + O2(g) + Br2(g) NOBr(g) from the
Question:
Determine Kc for the reaction
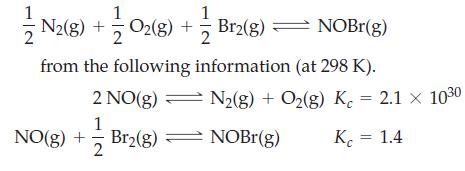
Transcribed Image Text:
2 N2(g) + O2(g) + — Br2(g) — NOBr(g) from the following information (at 298 K). 2 NO(g) 1 Br₂(g) NO(g) + 2 N2(g) + O₂(g) K = 2.1 × 10³⁰ NOBr(g) K = 1.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
To determine Kc for the reaction we can use the following equation Kc productsn reactantsm where n a...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
How is HRM technology being used to "strategically" recruit and staff? Share an example. How does screening software present challenges for recruitment and staffing? How does staffing HRM technology...
-
The equilibrium constant Kc for the reaction is 3.8 Ã 10-5 at 727°C. Calculate Kc and KP for the equilibrium at the same temperature. 12(g)--21(g) 21(g)- 2(g)
-
The equilibrium constant Kc for the reaction is 2.18 Ã 106 at 730°C. Starting with 3.20 moles of HBr in a 12.0-L reaction vessel, calculate the concentrations of H2, Br2, and HBr at...
-
Joe rents his condo for $1,500 per month. Total rental and personal use days for the current year was 210 days and 20 days, respectively. What are the tax consequences for Joe?
-
Jenny Rene, the CFO of Asor Products, Inc., has just completed an evaluation of a proposed capital expenditure for equipment that would expand the firms manufacturing capacity. Using the traditional...
-
Several years ago, Mary Emerson founded Emerson Consulting Inc., a consulting business specializing in financial planning for young professionals. The following captions and amounts summarize Emerson...
-
Remembering the target market segments you identified in Chapter 8 for your marketing plan:
-
Chocolates has observed the following overhead costs for the past 12 months: The results of the regression analysis are: TC = $8,781 + ($0:63 Ã Number of Boxes) a. Plot the data and the...
-
What is the effect on financial system and capital market for an negative interest rate.
-
A 0.0240 mol sample of N 2 O 4 (g) is allowed to come to equilibrium with NO 2 (g) in a 0.372 L flask at 25 C. Calculate the amount of N 2 O 4 present at equilibrium (Fig. 15-9). Figure 15-9 NO4(g) 2...
-
Ammonium hydrogen sulfide, NH 4 HS(s), used as a photographic developer, is unstable and dissociates at room temperature. A sample of NH 4 HS(s) is introduced into an evacuated flask at 25 C. What is...
-
Determine the moment of inertia of the area about the x axis. 1m y = x -1 m X
-
Using a ruler and set squares only, construct the following shapes: a. b. c. d. 5cm 5cm
-
The marketing department has just forecast that 10,000 units of item 778 will be ordered in the next fiscal year. Based on the marketing department's forecast and noting that the seasonal relative...
-
Following are interaction plots for three data sets. Which data set has the largest interactions? Which has the smallest? A B C
-
From your local chamber of commerce, obtain the population figures for your city for the years \(1980,1990,2000\), and 2010. Find the rate of growth for each period. Forecast the population of your...
-
A mass \(m\) is attached at the midpoint of a stretched wire of area of cross-section \(A\), length \(l\), and Young's modulus \(E\) as shown in Fig. 13.29. If the initial tension in the wire is...
-
The Coca-Cola Company's accounts receivable turnover was 9.05 in 2014, and its average amount of net receivables during the period was $3,424 million. What is the amount of its net credit sales for...
-
In the series connection below, what are the respective power consumptions of R, R2, and R3? R R www 4 V=6V P1-3 W; P2=3W; and P3= 3 W OP10.5 W; P2-1 W; and P3= 1.5 W P1=1.5 W; P2=1 W; and P3= 0.5 W...
-
Staircase Equipment Company uses a job order cost system. The following data summarize the operations related to production for April 2010, the first month of operations: a. Materials purchased on...
-
Lynch Furniture Company refinishes and reupholsters furniture. Lynch uses a job order cost system. When a prospective customer asks for a price quote on a job, the estimated cost data are inserted on...
-
Dacher Company uses a job order cost system. The following data summarize the operations related to production for October: a. Materials purchased on account, $450,000. b. Materials requisitioned,...
-
Yard Professionals Incorporated experienced the following events in Year 1, its first year of operation: Performed services for $31,000 cash. Purchased $7,800 of supplies on account. A physical count...
-
This question is from case # 24 of book Gapenski's Cases in Healthcare Finance, Sixth Edition Select five financial and five operating Key Performance Indicators (KPIs) to be presented at future...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...

Study smarter with the SolutionInn App


