A 1.05 mol sample of H 2 O(g) is compressed into a 2.61 L flask at 30.0
Question:
A 1.05 mol sample of H2O(g) is compressed into a 2.61 L flask at 30.0 °C. Describe the point(s) in Figure 12-30 representing the final condition.
Figure 12-30
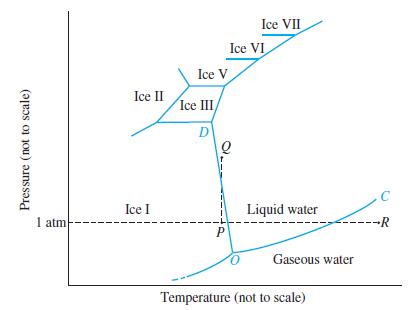
Transcribed Image Text:
Pressure (not to scale) 1 atm Ice II Ice I Ice V Ice III/ D P Ice VII Ice VI Liquid water Gaseous water Temperature (not to scale) C --R
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The point in Figure 1230 representing the final condition of the 105 mol sample of H2Og compressed i...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
When does self control begin, and how does it change as children's develop?
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Has Mississippi enacted the Uniform Real Property Electronic Recording Act? Provide the URL/web address of your source. Provide the citation to where this law is found in the Miss. Code (including...
-
On September 2, 2005, Levine executed a mortgage bond under which she promised to pay the Mykoffs a preexisting obligation of $54,000. On October 14, 2011, the Mykoffs transferred the mortgage to...
-
Lucas Contracting, Inc., is a small contractor in Carrollton, Ohio. Altisource Portfolio Solutions, Inc., hired Lucas to work on certain foreclosed properties. When payment for the work was not...
-
How can virtuous leadership contribute to healthcare management policies?
-
Matuska Tools Corporations income statements follow. Required 1. Prepare a multistep income statement for 2013 and 2014 showing percentages of net sales for each component (e.g., cost of goods sold...
-
Question 2 The risk premium is defined as the rate of return on: a U.S. Treasury bill. the overall market. a risky asset minus the risk-free rate. a risky asset minus the inflation rate. a riskless...
-
Gulf Uniform Supply, Inc. (GUS), a merchandising business, is organized as a corporation. The business sells a complete line of uniforms for a variety of businesses and organizations, including...
-
Because solid p-dichlorobenzene, C 6 H 4 Cl 2 sublimes rather easily, it has been used as a moth repellent. From the data given, estimate the sublimation pressure of C 6 H 4 Cl 2 (s) at 25 C For C 6...
-
To vaporize 1.000 g water at 20 C requires 2447 J of heat. At 100 C, 10.00kJ of heat will convert 4.430 g H 2 O(l) to H 2 O(g). Do these observations conform to your expectations? Explain.
-
Determine the angular velocity of the flywheel in Prob. 2257 which will produce an amplitude of vibration of 0.25 in. Prob. 2257 The electric motor turns an eccentric flywheel which is equivalent to...
-
Question 2: (40 points: 10 each) During September, Sweet Foods manufactures a single product. The Company's material purchases amounted to 9,000 pounds at a price of $9.80 per pound. Actual costs...
-
E12-23 (Algo) (Supplement 12B) Preparing a Statement of Cash Flows, Indirect Method: T-Account Approach [LO 12-S2] Golf Goods Incorporated is a regional and online golf equipment retailer. The...
-
A symmetric compound channel in over bank flow has a main channel with a bottom width of 30 m, side slopes of 1:1, and a flow depth of 3m. The floodplains on either side of the main channel are both...
-
Solve these question in details and fully explaination. It is the pre-lab working for Capacitors. Thanks so much in advance. 1: The figure shows a circuit with a charged capacitor (left), two...
-
Exercise 10-14A (Algo) Straight-line amortization of a bond discount LO 10-4 Diaz Company issued bonds with a $112,000 face value on January 1, Year 1. The bonds had a 8 percent stated rate of...
-
A compound is known to be a methyl anisole, but the orientation of the two substituents (-CH3 and -OCH3) on the aromatic ring is not known. The 13C NMR spectrum shows six peaks. Which isomer is it?...
-
For what reason might an exporter use standard international trade documentation (letter of credit, draft, order bill of lading) on an intrafirm export to its parent or sister subsidiary?
-
Calculating Interest Rates Solve for the unknown interest rate in each of thefollowing: Interest Rate Future Value Present Value $ 240 360 39.000 Years 2 10 15 307 896 174,384 483,500 38,261 30
-
Calculating the Number of Periods Solve for the unknown number of years in each of the following: Interest Rate 8% Future Value $ 1,284 Present Value $ 560 Years 4.341 364,518 A10 21 18 400 21.500...
-
Calculating Interest Rates Assume the total cost of a college education will be $280,000 when your child enters college in 18y. You presently have $50,000 to invest. What annual rate of interest must...
-
i just need anssers for G,h1,h2,h3 120 a. If the opportunity cost of capital is 11%, which of these two projects would you accept (A, B, or both)? b. Suppose that you can choose only one of these two...
-
In using Verizon Communications Inc as a case analysis, what is their product portfolio, competitors and competitive Environment?/
-
Tony and Suzie graduate from college in May 2021 and begin developing their new business. They begin by offering clinics for basic outdoor activities such as mountain biking or kayaking. Upon...

Study smarter with the SolutionInn App


