Question: (A) 131 I is a emitter used as a tracer for radioimmunoassays in biological systems. Use information in Table 25.1 to determine (a)
(A) 131I is a β– emitter used as a tracer for radioimmunoassays in biological systems. Use information in Table 25.1 to determine
(a) The decay constant in s–1;
(b) The activity of a 2.05 mg sample of 131I;
(c) The percentage of 131I remaining after 16 days;
(d) The rate of β– emission after 16 days.
(B) 223Ra has a half-life of 11.43 days. How long would it take for the activity associated with a sample of 223Ra to decrease to 1.0% of its current value?
Table 25.1
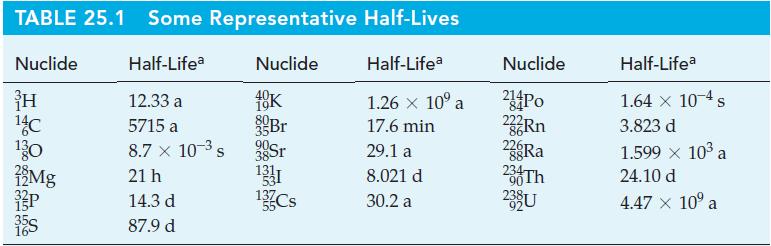
TABLE 25.1 Some Representative Nuclide Half-Lifea Nuclide H 12.33 a 10K 14C 5715 a 30 8.7 x 10- s 21 h 14.3 d 87.9 d Mg Br 3 Sr 131 13 Cs Half-Lives Half-Lifea 1.26 x 10 a 17.6 min 29.1 a 8.021 d 30.2 a Nuclide 214 Po 84 222Rn 86 22 Ra 88 234 Th 90 239 U Half-Lifea 1.64 x 10-4 s 3.823 d 1.599 10 a 24.10 d 4.47 10 a
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
A 131 Decay a Decay Constant The decay constant is related to the halflife t by the equation In2 For ... View full answer

Get step-by-step solutions from verified subject matter experts


