(A) For reaction (20.3), determine (a) The instantaneous rate of reaction at 2400 s and (b) [H...
Question:
(A) For reaction (20.3), determine
(a) The instantaneous rate of reaction at 2400 s and
(b) [H2O2] at 2450 s.
Assume that the instantaneous rate of reaction at 2400 s holds constant for the next 50 s.
Reaction (20.3)
![]()
(B) Use data only from Table 20.2 to determine [H2O2] at t = 100 s. Compare this value with the one calculated in Example 20-2(b). Explain the reason for the difference.
Table 20.2
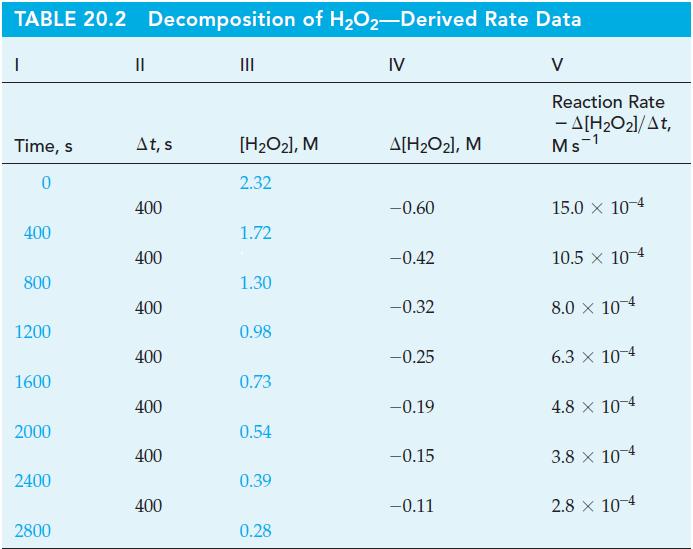
Example 20-2(b)
From the data in Table 20.2 and Figure 20-2 for the decomposition of H2O2, determine
(b) [H2O2]t at t = 100 s, assuming that the initial rate is constant for at least 100 s.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





